Organic Chemistry : Some Basic Principles And Techniques Class 11 Notes Chemistry Chapter 8 - CBSE
Chapter : 8
What Are Organic Chemistry : some Basic Principles And Techniques ?
Organic Chemistry
It is the branch of chemistry that deals with the study of hydrocarbons and their derivative.
The Shapes Of Carbon Compounds
The formation and the shapes of molecules like methane (CH4), ethene (C2H4), ethyne (C2 H2) are explained in terms of the use of sp3 , sp2 and sp hybrid orbitals by carbon atoms in the respective molecules.
Hybridisation influences the bond length and bond enthalpy (strength) in organic compounds.
Some Characteristic Features Of π Bonds
- π-bonds is formed by sideways or lateral over-lapping of parallel p-orbitals.
- The p-orbitals should be parallel and perpendicular to the plane of molecule.
- Rotation around one CH2 fragment with respect to other interferes with maximum overlap of p-orbitals and therefore, such rotation of C=C is restricted, which leads to formation of geometrical isomers i.e., cis transisomers in alkenes.
Structural Representation Of Organic Compounds
The structure of an organic compound can be represented using any one of the below mentioned methods.
- Lewis structure or dot structure
- Dash structure or line bond structure
- Condensed structure
- Bond line structure
| Molecular Formula | Condensed Structure | Bond Line Structure |
| n-propanol C3H2O | CH3 –CH2–CH2–OH | 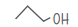 |
| 1, 3-butadiene C6H6 | CH2=CH–CH–CH2 | 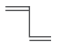 |
| t-butyl chloride C4H9Cl | 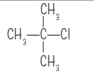 |
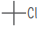 |
| 1,3-dimethyl cyclopentane C2H16 |  |
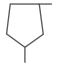 |
Classification Of Organic Compounds
Organic Compounds
- Acyclic or Open Chain Compounds (I)
- Cyclic or Closed Chain or Ring Compounds (II)
- Homocyclic or Carbocyclic Compounds
- Alicyclic Compounds
- Aromatic Compounds
- Benezenoid Compounds
- Non-benezenoid Compounds
- Heterocyclic Compounds
- Homocyclic or Carbocyclic Compounds
Acyclic Or Open Chain Compound
They consist of straight or branched chain compounds,

Alicyclic or closed chain or ring compounds
They contain carbon atoms joined in the form of a ring (homocyclic) or atoms other than carbon are also present in the ring (heterocylic).

Aromatic compounds
Some of the examples of various types of aromatic compounds are:
Benezenoid Aromatic Compounds

Non-benezenoid Compounds

Heterocyclic Aromatic Compounds

Functional Group
The functional group are atom or group of atoms joined in a specific manner which determines the chemical properties of the organic compound. The examples are hydroxyl group (—OH), aldehyde group (—CHO) and carboxylic acid group (—COOH) etc.

Homologous Series
A group or a series of organic compounds each containing a characteristic functional group forms a homologous series and the members of the series are called homologues. The members of a homologous series can be represented by general molecular formula and the successive members differ from each other in molecular formula by a –CH2 unit. Some of homologous series of organic compounds are alkanes, alkenes, alkynes, haloalkanes, alkanols, alkanals, alkanones, alkanoic acids, amines etc.
Nomenclature Of Organic Compounds
Common name (Common system): Before the IUPAC system of nomenclature, organic compounds were named after the sources of origin, for example, urea was so named because it was obtained from the urine of mammals. Formic acid was so named since it was extracted from red ants called formica.
Common or Trivial Names of Some Organic Compounds
| Compound | Common Sense | Compound | Common Sense |
| CH4 | Methane | CHCl3 | Chloroform |
| H3 CCH2CH2CH3 | n-Butane | CH3COOH | Acetic Acid |
| (H3C)2CHCH3 | isobutane | C6H6 | Benzene |
| (H3C)4C | Neopentane | C6H5OCH3 | Anisole |
| H3CCH2CH2OH | n-Propyl alcohol | C6H5NH2 | Aniline |
| HCHO | Formaldehyde | C6H5COCH3 | Acetophenone |
| (H3C)2CO | Acetone | CH3OCH2CH3 | Ethyl methyl ether |
IUPAC (International Union Of Pure And Applied Chemistry) System
According to IUPAC system, the name of an organic compound contains three parts:
Word root
Word root represents the number of carbon atoms present in the principal chain, which is the longest possible chain of carbon atoms. For special word roots: meth-C1 , eth-C2 , prop-C3 , but-C4 ,
Suffix
- Primary Suffix: It indicates the type of bond in the carbon atoms. For example: and for ane C—C bond, ene
C= C bond, yne C≡ C bond. - Secondary Suffix: Secondary suffix is used to represent the functional group.
Prefix
Prefix is a part of IUPAC name which appears before the word root. Prefix are of two types:

- Primary prefix: For example, primary prefix cyclo is used to differentiate cyclic compounds.
- Secondary prefix: Some functional groups are considered as substituents and denoted by secondary prefixes. For example: –F Flupro, –Cl Chloro, –Br Bromo, –NO Nitroso, –NO2Nitro, –CH3 Methyl, –OCH3 Methoxy.
Naming Of Compounds Containing Functional Groups
The longest chain of carbon atoms containing the functional group is numbered in such a manner that the functional group is attached at the carbon atoms possessing lowest possible number in the chain.
Polyfunctional Compounds
In case of polyfunctional compounds, one of the functional group is selected as principal functional group and the compound is named on that basis. The choice of principal functional group is made on the basis of
order of preference. The order of decreasing priority for the functional group is:
–COOH, –SO3 H, –COOR (R= alkyl group) COCl, –CONH2 , COCl,
–CONH2 , –CN, –C =O, > –C= O, –OH, –NH2 > C ≡C<, –C≡ C–
Isomerism
The phenomenon of existence of two or more compounds possessing the same molecular formula but different properties is known as isomerism. Such compounds are called as isomers.
Structural Isomerism
It is shown by compounds having the same molecular formula but different structural formulae differing in the arrangement of atoms.
- Chain Isomerism
- Position Isomerism
- Functional Group Isomerism
- Metamerism
Stereoisomerism
- Geometrical Isomerism
- Optical Isomerism
Fundamental Concepts In Organic Reaction Mechanism
A sequential account of each step, describing details of electron movement, energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products (kinetics) is referred to as reaction mechanism. The general reaction is depicted as follows:
$$\text{Organic molecule (Substrate)}\\\xrightarrow{\text{Attacking Reagent}}\\{ [\underset{\dashrightarrow\text{By Products}}{\text{Intermediate}]}}\xrightarrow{}{\text{Products(s)}}$$
Fission Of A Covalent Bond
A covalent bond can undergo Fission in two ways:
- Homolytic Fission: In this process each of the atoms acquires one of the bonding electrons.
$$\text{A–B or A : B }——›\text{A}^{.} + \text{B}^{.}$$
- Heterolytic Fission: In this process one of atoms acquires both of the bonding electrons when the bond is
broken. If B is more electronegative than A which thereby acquires both the bonding electrons and becomes
negatively charged.
The products of heterolytic fission are ions. Heterolytic and homolytic bond fission results in the formation of
short-lived fragments called reaction intermediates. Among the important reaction intermediates are
carbonium ions, carbanions, carbon free radicals and carbenes. Carbonium Ions (carbocations) are organic
ions which contain a positively charged carbon atom. They are formed by heterolytic bond fission.

Where, Z is more electronegative than carbon. Tertiary carbonium ion is more stable than a secondary, which in turn is more stable than a primary because of +I effect associated with alkyl group.

- Carbanion are organic ion which contains a negatively charged carbon atom. They are also formed by heterolytic bond fission.

Where, Z is less electronegative than carbon. A primary carbanion is more stable than a secondary, which in turn is more stable than a tertiary, because of +I effect associated with alkyl group.
- Electrophile is positively charged or neutral species which is electron deficient, e.g., H20+ , CH3 , NH4+ , AlCl3 ,S03, CHCl2 .
- Nucleophile is negatively charged or neutral species with lone pair of electrons e.g., (HO–), Cyanide (C = N), H20: R3 N, R2 NH etc.

Electron Displacement Effects In Covalent Bond
Electronic displacements in covalent bonds occurs due to the presence of an atom or group of different electronegativity or under the influence of some outside attaching group. These lead to a number of effects
which are as follows:
Inductive Effect
The σ electrons which form a covalent bond are seldom shared equally between the two atoms. Due to different electronegatively electrons are displaced towards the more electronegative atom. This introduces a certain degree of polarity in the bond. The more electronegative atom acquires a small negative charge (δ–). The less electronegative atom acquires a small positive charge (δ+).

- Structure I- indicates the relative charges on the two atoms.
- Structure II- indicates the direction in which the electrons are drawn.
Electromeric Effect (Effect)
It refers to the polarity produced in a multiple bonded compound when a double or a triple bond is exposed to an attack by an electrophile E+ (a reagent). The electromeric effect is represented as:
The curved arrow shows the displacement of the electron pair. The atom A has lost its shared pair of electron and B has gained that shared pair of electron. Therefore, A acquires a positive charge and B a negative charge.
Resonance Structure
The resonance structures (canonical structures or contributing structures) are hypothetical and individually do not represent any real molecule. For example, benzene is ordinarily represented as: This structure has three C–C bonds and three C ≡ C bonds.

The structure of benzene cannot be represented by single structure. It can be represented equally well by the energetically similar structures I and II. The two structures are called resonance structures.

Actual structure of benzene is resonance hybrid of structures I and II.
Hyperconjugation or No Bond Resonance
When the alkyl group is attached to an unsaturated system such as —CH=CH2 group the order of inductive effect gets reversed. The behaviour can be explained by hyperconjugation effect. Such structures are arrived at by shifting the bonding electrons from an adjacent C —H bond to the electron deficient carbon. In this way, the positive charge originally on carbon is dispersed to the hydrogen. This way of electron release by assuming no bond character in the adjacent C—H bond is called No-Bond Resonance or Hyperconjugation.

Orbital Concept Of Hyperconjugation
It involves delocalisation of o electrons of C—H bond of an alkyl group which is attached directly to an atom of unsaturated system or to an atom with an unshared p-orbital. In general, greater the number of alkyl groups attached to a positively charged carbon atom, the greater is the hyperconjugation.
Types Of Organic Reactions
Organic reactions can be classified into the following categories:
Substitution Reactions
The direct replacement (displacement or substitution) of an atom or group of atoms in an organic molecule by another atom or group of atoms without any alteration in the remaining part of the molecule is known as a substitution reaction.
Types of Substitution Reaction
Depending on the nature of the attacking reagent, substitution reactions are further categorized into three categories :
- Nucleophilic substitution reaction: In these reactions, the attacking reagent is a nucleophile (Nu– or Z– ). These reactions are typically of alkyl halides.

$$\underset{\text{Alcohol (Substitution product)}}{\text{R - OH}} +\underset{\underset{\text{(Weaker nucleophile)}}{\text{Halide ion}}}{\text{X}^{\normalsize-}}\\\text{(X = Cl, Br or I)}$$
- Electrophilic Substitution Reactions: In these reactions, the attacking reagent is an electrophile. These reactions are typical of arenes and other aromatic compounds. For example, halogenation, nitration,
sulphonation, and Friedel-Crafts reactions. - Free Radical Substitution Reaction: In these reactions, the attacking reagent is a free radical. These
reactions take place at high temperatures or in the presence of UV radiation. For example, chlorination of
methane to form chloromethane.
Addition Reaction
They are defined as reactions in which two reactive molecules combine to form a single product molecule. Compounds with many (double and triple) bonds are susceptible to such reactions.
Addition reactions are classified into three categories based on the nature of the attacking species. They are:
- Electrophilic Addition Reaction: These are addition reactions caused by electrophiles. These are typical alkene and alkyne reactions. For example, the addition of halogen acids to alkenes.
- Nucleophilic Addition Reaction: These are addition reactions that are caused by nucleophiles. These are typical aldehyde and ketone reactions. For example, base-catalysed addition of HCN to aldehydes or ketones.
- Free Radical Addition Reaction: These are addition reactions caused by free radicals. For example, addition of HBr to alkenes in the presence of peroxides
$$\underset{\text{Propene}}{\text{CH}_{3}\text{CH}=\text{CH}_{2} + \text{HBr}_{2}}\xrightarrow{\text{Peroxide}}\\\underset{\text{n-Propyl bromide}}{\text{CH}_{3}-\text{CH}_{2}-\text{CH}_{2}\text{Br}} $$
Elimination Reactions
The reaction in which two atoms or groups either from the adjacent positions or from the same position get eliminated or removed, leading to the formation of multiple bonds (i. e., double or triple bond) is known as an
elimination reaction. These reactions are of two types. They are;
- α– Elimination reactions: In these reactions, there is a loss or elimination of two atoms or groups from the
same carbon atom in the molecule. For example, Dehydrogenation of primary or secondary alcohols with reduced copper at 573k - β– Elimination reactions: In these reactions, the loss of two atoms or groups takes place from the adjacent carbon atoms in the molecule. For example, Dehydration of alcohols in the presence of concentrated sulphuric acid.
Rearrangement Reactions
Reactions involving the migration of an atom or a group from one atom to another within the same molecule are called rearrangement reactions. Wohler’s synthesis of urea from ammonium cyanate is also an example
of a rearrangement reaction.
$$\text{NH}_{4}\text{Cl} + \text{NaCNO}\xrightarrow{\Delta}\text{NH}_{4}\text{CNO} + \text{NaCl}$$
Share page on
Chapterwise Notes Class 11 Chemistry
CBSE CLASS 11 Notes
- CBSE Class 11 Physics Notes
- CBSE Class 11 Chemistry Notes
- CBSE Class 11 Maths Notes
- CBSE Class 11 Biology Notes
- CBSE Class 11 Accountancy Notes
- CBSE Class 11 Business Studies Notes
- CBSE Class 11 Economics Notes
- CBSE Class 11 History Notes
- CBSE Class 11 Geography Notes
- CBSE Class 11 Political Science Notes
- CBSE Class 11 Entrepreneurship Notes
CBSE CLASS 11 SYLLABUS
- CBSE Class 11 English Core Syllabus
- CBSE Class 11 Mathematics Syllabus
- CBSE Class 11 Physics Syllabus
- CBSE Class 11 Chemistry Syllabus
- CBSE Class 11 Biology Syllabus
- CBSE Class 11 Accountancy Syllabus
- CBSE Class 11 Business Studies Syllabus
- CBSE Class 11 Economics Syllabus
- CBSE Class 11 History Syllabus
- CBSE Class 11 Geography Syllabus
- CBSE Class 11 Sociology Syllabus
- CBSE Class 11 Political science Syllabus
- CBSE Class 11 Psychology Syllabus
- CBSE Class 11 Physical Education Syllabus
- CBSE Class 11 Applied Mathematics Syllabus
- CBSE Class 11 History of Indian Arts Syllabus
