Metals And Non-Metals Class 10 Notes Chemistry Science Chapter 3
Introduction
Around 114 total elements were present in the periodic table. As of 2016, the periodic table has 118 confirmed elements, from element-1 (Hydrogen) to 118 (oganesson).
Elements: (4) new elements were officially confirmed by the International Union of Pure and Applied Chemistry (IUPAC) in December 2015.
Total Elements : 114 (4-new)
Total Metal : 88
Total Non-metal : 22
Total Metalloids : 8
Physical properties of metals and non-metals
| Property | Metals | Non-metals |
| State | Metals are Solids. (except Mercury) | Non metals may be Solids, Liquids or Gases. (Bromine is the only non-metal in liquid state) |
| Hardness | Metals are Hard. (except Lithium, Potassium, Sodium) | Non - metals are Soft. (except Diamond which is the Hardest natural substance) |
| Luster | Metals have Metallic Luster. (Shine) | Non metals do not have Luster. (except Iodine crystals). |
| Malleability | Metals are Malleable. (Can be Beaten into Thin Sheets) | Non metals are Not Malleable . |
| Ductility | Metals are Ductile. (can be converted into thin wires) | Non metals are Not Ductile. |
| Melting point | Metals have High Melting Points. (Gallium and Cesium have low melting points. They melt in the palm of the hand) | Non metals which are Solids have Low Melting Points. |
| Boiling point | Metals have High Boiling Points. | Non metals which are Solids and Liquids have Low Boiling Points. |
| Conductor of heat and electricity | Metals are Good Conductors of Heat and electricity. (Best conductors are silver and copper) (Poor conductors are Lead and Mercury) | Non metals are Bad Conductors of Electricity . (Except Graphite) |
| Sonority | Metals are Sonorous. (Produces sound when beaten) | Non metals are Not Sonorous. |
- Noble Metal: Noble metals are metals that are resistant to corrosion or oxidation, unlike most base metals. They tend to be precious metals, often due to perceived rarity. Ex: Tantalum, Gold, Platinum, Rhodium.
- Precious Metal: These are rare metallic chemical elements of high economic value. Chemically the previous metals are less reactive than most elements, have high luster and high electrical conductivity. Ex: Gold, Copper (coinage elements) Platinum (most widely traded).
- Base Metals: A metal that oxidises or corrodes relatively easily and reacts variably with dilute (HCl) to form hydrogen. Ex: Iron, Nickel, Lead, Zinc.
Chemical properties of metals -
| Property | Metals | Non-metals |
| Nature | Metals show the tendency to lose valence electrons, to form positive ions (Cations). Hence, electropositive in nature. [Na→Na+ + e–] |
Non-metals show the tendency to gain electrons to complete its octet , to form negative ions (anions). Hence electronegative in nature. [Cl + e– → Cl–] |
| Reaction with water | Metals react with Water to form Metal Oxides or Metal Hydroxides and Hydrogen. 2Na(s)+2H2O(l) → 2NaOH(aq)+H2(g) 2Al(s)+3H2O(l) → Al2O3(aq)+3H2(g) |
Do not react |
| Reaction with Oxygen | Metals react with Oxygen present in the air to form Metal Oxides (ionic oxides). 2Mg(s) + O2(g) → 2MgO(s) Metallic oxides are generally basic or amphoteric in nature. |
Non-Metals react with Oxygen present in the air to form non-Metal Oxides (covalent oxides). C(s) + O2(g) → CO2(g) Non-Metallic oxides are generally acidic or neutral in nature. |
| Reaction with dilute acids | Metals react with Dilute Acids to form Salts and Hydrogen gas. Metals displace hydrogen of Dilute Acids to form metal salts and Hydrogen gas. Mg(s)+2HCl(aq) → MgCl2(aq)+H2(g) |
No reaction with dilute acids. They react with strong acids: C + 2H2SO4 → 2H2O + CO2 + SO2 |
| Reaction of Metals with Metal Salt solutions | A more reactive metal displaces a less reactive metal from its salt solution (Displacement reaction). Metal A + Salt Solution of Metal B → Salt Solution of Metal A + Metal B Fe(s)+CuSO4(aq)→FeSO4(aq)+Cu(s) |
They do not react with salt solution but displaces less reactive non-metal from the salt. |
- At room temperature, sodium is soft and can be cut with a knife. When exposed to humid air, the silver white surface quickly oxidises. Hence to protect them from accidental fires, they are kept immersed in kerosene oil.
- Some metals like Magnesium, Aluminium, Zinc, Lead etc. forms an Oxide Layer over it which Prevents further Oxidation. They are called Self Protecting Metals or passive metals.
- Amphoteric oxides - Those metal oxides which show basic as well as acidic behaviour are known as amphoteric oxides. Aluminium metal and zinc metal form amphoteric oxides. Thus, aluminium oxide and zinc oxide are amphoteric in nature (which show basic as well as acidic behaviour).
- Fe does not burn on heating but iron fillings burn vigorously when sprinkled in the flame of burner
- If concentrated HNO3 is simultaneously added to copper, it reacts immediately and produces brown fumes of NO2.
- Acids are often used to dissolve metals or organic material. However, certain materials are more difficult to dissolve. They require more potent and specialized acid solutions like aqua regia to dissolve gold and platinum.
Reactivity series
On the basis of reactions of some important metals with oxygen, water and acids, as well as displacement reactions, the metals have been arranged in a group or series according to their chemical reactivity.
This arrangement of metals in a vertical column in decreasing reactivity order is known as a reactivity series of metals (or activity series of metals).
| Order of reactivity | Reaction with water | Reaction with dilute acid |
| Potassium | Fizz, given off hydrogen gas, Leaving an alkaline solution of metal hydroxide | explode |
| soduim | ||
| lithium | ||
| calcium | fizz, givin off hydrogen gas and forming a salt | |
| magnesium | very slow reaction | |
| aluminium | ||
| zinc | ||
| iron | ||
| tin | slight reaction with steam | react slowly with warm acid |
| lead | ||
| copper | no reaction, even with steam | no reaction |
| silver | ||
| gold |
Ionic or electrovalent bond
The bond formed between a metal and a nonmetal, by a transfer of electron(s), is called an Ionic or Electrovalent bond.
Formation of NaCl (Sodium Chloride)
Metals : Loses electrons and becomes Positive ions.
$$\underset{\underset{}{\textbf{(2,8,1)}}}{Na_{(s)}}\xrightarrow[]{} \underset{\underset{}{\textbf{(2,8)}}}{Na^+}+1e^-$$
Non - Metals :- Gains electrons and becomes Negative ions.
$$\underset{\underset{}{\textbf{(2,8,7)}}}{Cl_{(g)}}+e^-\xrightarrow[]{} \underset{\underset{}{\textbf{(2,8,8)}}}{^-Cl}$$
$$\underset{\underset{}{\textbf{(2,8)}}}{Na^+}+\underset{(2,88)}{Cl^-}\xrightarrow[]{} \underset{\underset{}{\textbf{Sodium Chloride}}}{NaCl}$$

Properties of ionic compounds
- Physical Nature of Ionic Compounds :
- Solids , Somewhat Hard due to strong inter-particle forces of attraction.
- Generally Brittle and break under pressure. - Ionic Compounds are :
- Formed by the Transfer of Electrons.
- Made up of ions. - Ionic Compounds are Crystalline Solids.
For example NaCl is a crystalline solid. - Solubility of Ionic Compounds :
- Soluble in Water and Insoluble in Organic solvents (like petrol, kerosene etc.)
For example, copper sulphate is a blue colored ionic compound, which when added to water, dissolves in water to form a blue coloured copper sulphate solution. - Ionic Compounds have :
- High Melting points
- High Boiling points. - They Conduct Electricity
- In Molten State or In Solution.
Sodium Chloride solution conducts electricity in aqueous or molten state.
Basic metallurgical processes
Mineral : Those compounds which occur naturally in earth's crust.
Ores : Those minerals from which metal can be extracted out profitably and conveniently.
Gangue (Matrix) : The unwanted impurities present with ore.
Metallurgy: It is the branch of science which deals with the extraction of metals from their ores and then refining them for use.
Steps in Metallurgy
- Concentration of the Ore ( Enrichment of the ore ).
Objective : To obtain pure ore, by removal of Gangue from the ore. - Reduction to the metal.
Objective : To obtain the Metal from the ore. - Refining ( Purification of the metal ).
Objective : To obtain Pure Metal from the impure metal by removal of impurities.
Activity series and related metallurgy

Extraction of Metals from their Ores
Extraction of metals Low in the Activity Series
Metals which are low in the activity series can be reduced to the metals by heating in the presence of oxygen (Roasting).
- Mercury is obtained from its ore Cinnabar (HgS) by heating in the presence of
When it is heated in the presence of oxygen it is first converted into Mercuric Oxide (HgO). On further heating it is reduced to Mercury (Hg).
2HgS(s) + 3O2(g) ⟶ 2HgO(s) + 2SO2(g)
2HgO(s) ⟶ 2Hg(l) + O2(g)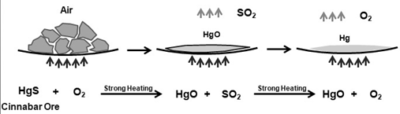
- Copper is obtained from its Sulphide ore (Cu2S) by heating in the presence of
2Cu2S(s) + 3O2(g) ⟶ 2Cu2O(s) + 2SO2(g)
2Cu2O(s) + Cu2S(s) ⟶ 6Cu(s) + SO2(g)
Extraction of metals middle in the Activity Series
Metals in the middle of the activity series- Zinc, Lead, Iron
Oxidation of metal ores
Ores of these metals generally exist as oxides, Sulphides, and carbonates.
It is easier to obtain metals from their Oxides than from their Sulphides or carbonates. Therefore, at first non-oxide ores are converted into oxides ores by calcination or roasting.
| Calcination | Roasting |
| It is the process of converting an ore into its oxides by heating it strongly, below its melting point in limited supply of air or in absence of air. | It is the process of converting an ore into its metallic oxide by heating it strongly below its melting point in excess of air. |
| This process is commonly used for carbonate ores. | This process is commonly used for sulphide ores and is carried out in blast furnaces. |
Reduction of oxide to metal
The oxide ore is then reduced to the metal by heating with a reducing agent. The most common reducing agent is coke (carbon).
ZnO(s) + C(s) ⟶ Zn(s) + CO(g)
Removal of Oxygen from a compound is called Reduction and the substance which removes Oxygen from the compound is called Reducing Agent .
Extraction of metals top in the Activity Series
The highly active metals at the top of activity series cannot be obtained from their ores by −
- Simple
- Heating with Reducing Agents.
They are obtained by Electrolytic Reduction of their Molten Chlorides. Example :
When electric current is passed through Molten Sodium Chloride,
- Sodium Metal is deposited at the Cathode.
- Chlorine gas is liberated at the Anode.
$$\textbf{At Anode:}\space 2Cl^-(aq) - 2e^-→ Cl_2↑\\\textbf{At Anode:}\space Na^+(aq) + e^-→ Na_{(s)}$$
Refining of metals
The Removal of Impurities from the metal to obtain the pure metal is called as Refining of Metals.
Electrolytic refining of metals
Method -
- A block of the Impure metal is made the Anode
- A thin sheet of Pure Metal is made of Cathode.
- The Electrolyte is a salt solution of the metal to be purified.
For example - electrolytic refining of copper
A block of impure copper is made the anode and a thin sheet of pure copper is made the cathode.
The Electrolyte is an acidified Copper Sulphate solution. When electric current is passed through the electrolyte, Pure Copper from the Anode is deposited at the Cathode and the Impurities settle down as Anode Mud.
$$\textbf{At Anode:}\space Cu_{(s)} - 2e^-→Cu^{2+}\\\textbf{At Anode:}\space Cu^{2+}(aq) + 2e^-→ Cu_{(s)}$$
Below Anode: AnodeNud
Corrosion -
Corrosion is the damage caused to Metals due to the reaction of Metals with Oxygen, Moisture, Carbon Dioxide, etc.
Conditions Necessary for Corrosion -
- Presence of air (or Oxygen)
- Presence of water (or Moisture)
Corrosion of metals can be prevented by
- Applying oil or grease.
- Applying paint.
- By Galvanization. (Coating with zinc)
[Galvanizing is a process by which molten zinc is sprayed on the surface of iron or by immersing the iron object in a bathtub containing molten zinc.] - By tinning. (Coating with tin)
[Tinning is the process of thinly coating the sheets of wrought iron or steel with tin, and the resulting product is known as tinplate. It is most often used to prevent rust.] - By alloying. (Making alloys)
[An Alloy is a homogeneous mixture of two or more metals or a metal and a nonmetal.]
- Metals resistant to Corrosion are Gold, Platinum, and Titanium.
- Important Alloys and their composition - Duralumin - (Al , Mg , Mn , Cu )
Magnalium - (Al , Mg )
Brass- (Cu, Zn )
Bronze - (Cu , Zn, Sn )
German Silver - (Cu , Zn, Ni )
Stainless steel - (Fe, C, Ni, Cr)
Nickel Steel - (Fe, C, Ni )
Alnico - (Al, Fe, Ni , Co)
What are Metals and Non-metals?
- Elements are classified into Metals, Non-metals and Metalloids
Note :
- (1) Copper oxide is black in colour.
- (2) Aluminium oxide is amphoteric oxide.
- (3) Sodium and potassium react violently with water
- (4) Magnesium do not react with cold water.
- (5) Some metals like aluminium, iron and zinc do not react either with cold or hot water.
| 1. Metals: A substance with high electrical conductivity, lustre and malleability which readily loses electrons to form positive ions. | 11. Electrodes: An electrode is a solid electric conductor that carries electric current into non-metallic solids, or liquids, or gases, or plasmas, or vacuums. |
| 2. Non-metals: A substance with low electrical conductivity, non-lustrous, non-malleable and which readily gains electrons to form negative ions. | 12. Mineral: A mineral is a naturally occurring solid with a characteristic composition, crystalline atomic structure and distinct physical properties. |
| 3. Metalloids: Elements with properties intermediate between those of a metal and non-metal. | 13. Smelting: It is a chemical process to isolate an element from its ore using heat and a reducing agent. |
| 4. Alloy: A homogenous mixture of a metal with at least one other metal or non-metal. | 14. Gangue: It is an unwanted material or impurities in the form of sand, rock or any other material that surrounds the mineral in an ore deposit. |
| 5. Malleability: It is a physical property of metals that defines the ability to be hammered, pressed, or rolled into thin sheets without breaking. | 15. Calcination: The conversion of metals into their oxides as a result of heating to a high temperature in the absence of air or oxygen. The organic matter, moisture, volatile impurities like carbon dioxide and sulphur dioxide are expelled from the ore which makes the ore porous. |
| 6. Sonorous: It is the property of a substance to produce a deep resonant sound when collide together. | 16. Roasting: It is a process in metallurgy in which a sulphide ore is heated in air. The process may convert a metal sulphide to a metal oxide or to a free metal. |
| 7. Ductility: It is a physical property of a material associated with their ability to be hammered thin or stretced into a wire without breaking. | 17. Electroplating: A process that uses electric current to reduce dissolved metal cations so that they form a thin coherent metal coating on an electrode. |
| 8. Ore: A naturally occurring solid material from which a metal or valuable mineral can be extracted profitably. | 18. Galvanisation: A process that applies a coat of zinc to a metal to prevent its oxidation. A process that applies a coat of zinc to a metal to prevent its oxidation. |
| 9. Anion: A negatively charged ion formed by gain of electrons. | 19. Refining: It is a method of removing impurities in order to obtain metals of high purity. |
| 10. Cation: A positively charged ion formed by loss of electrons from a neutral atom. | |
