Among the World Ozone Day essay writing topics, ozone layer depletion and its measures is an essential one. Drafting an ozone layer depletion essay requires us to first understand what is an ozone layer.
The ozone layer is a region of Earth’s stratosphere containing relatively high ozone concentration (O3) molecules. The ozone layer plays a significant role in protecting life on Earth by absorbing and blocking a significant portion of the sun’s harmful ultraviolet (UV) rays. This absorption of UV radiation helps protect living organisms from the harmful effects of excessive UV exposure, like skin cancer, cataracts and damage to ecosystems.
Human activities, particularly the release of certain chemicals called ozone-depleting substances, have led to the thinning of the ozone layer in some regions, resulting in the ozone hole.
In this ozone layer depletion essay, we will discuss the various factors contributing to the depletion and amendments that can be adopted to control the same.
- ▪ What is an Ozone Layer?
- ▪ Causes of Ozone Layer Depletion
- ▪ Consequences of Ozone Layer Depletion
- ▪ Global Initiatives to Fight Ozone Layer Depletion
- ▪ Future Predictions and Steps Forward
- ▪ Role of Man in Protecting Ozone Layer
- ▪ Debunking the Myths on Ozone Layer Depletion
- ▪ Wrapping Up
- ▪ FAQs on Ozone Layer Depletion Essay

What is an Ozone Layer?
One of the major components in an ozone layer depletion essay is the composition of this layer and understanding where it is located. Let’s move ahead!
The ozone layer is primarily composed of ozone molecules. It is situated in the Earth’s stratosphere. This layer forms naturally in the stratosphere when oxygen molecules (O2) are exposed to high-energy ultraviolet (UV-C) radiation, causing them to split into individual oxygen atoms. These oxygen atoms can then react with other oxygen molecules to form ozone (O3). This continuous ozone creation and destruction maintains the ozone layer’s balance.
The ozone layer protects the Earth by absorbing and blocking a significant portion of the sun’s harmful ultraviolet radiation. Here’s how it works:
1. Absorption of UV-B and UV-C radiation: The ozone molecules in the ozone layer are particularly effective at absorbing UV-B and UV-C radiation, which are high-energy forms of ultraviolet light emitted by the sun. When these high-energy UV rays strike ozone molecules, they are absorbed and their energy is converted into heat.
2. Preventing harmful UV radiation from reaching the Earth’s surface: By absorbing and dispersing UV-B and UV-C radiation, the ozone layer acts as a shield, preventing a significant portion of these harmful rays from reaching the Earth’s surface. This is crucial because excessive exposure to UV radiation can have harmful effects on living organisms, including humans.
3. Protection against health risks: The ozone layer’s protective role is vital for safeguarding human health. UV-B radiation, for example, is known to cause various health issues such as sunburn, skin cancer, cataracts, and immune system suppression. If not absorbed by the ozone layer, UV-C radiation could be extremely harmful, but fortunately, it is almost entirely absorbed by the ozone molecules.
Long thing short, the ozone layer acts as a natural sunscreen for the Earth, absorbing and blocking harmful UV radiation from the sun, which helps prevent a range of health and environmental risks linked with prolonged UV exposure.
Causes of Ozone Layer Depletion
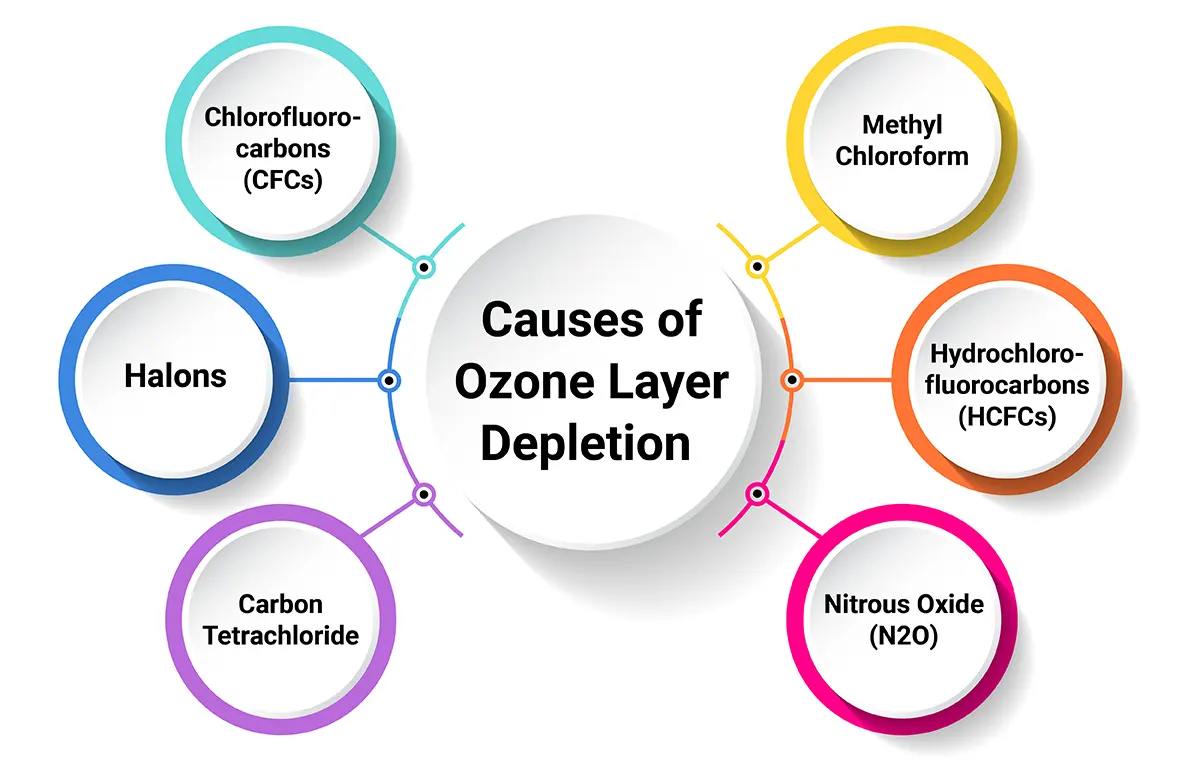
In this ozone layer depletion essay, it’s important to note the various causes that are resulting in ozone depletion. Causes are both natural and man-made, and now, let’s have a look at them.
Manmade causes of ozone layer depletion primarily involve the release of certain chemicals into the atmosphere, which can break down ozone molecules. The most significant contributors include:
1. Chlorofluorocarbons (CFCs) are a major reason for depletion: CFCs were commonly used in refrigerants, aerosol propellants and foam-blowing agents. When released into the atmosphere, they break down in the stratosphere, releasing chlorine atoms that destroy ozone molecules.
2. Halons: Among the major causes of Ozone layer depletion is the use of Halons. These were used in fire extinguishers. Like CFCs, they release bromine and chlorine when they break down, which are ozone-depleting substances.
3. Carbon Tetrachloride: This chemical is used in the production of CFCs and as a solvent. It releases chlorine when it breaks down.
4. Methyl Chloroform: It is used as a solvent, and in industrial processes, it releases chlorine, contributing to ozone layer depletion.
5. Hydrochlorofluorocarbons (HCFCs): While less harmful than CFCs, HCFCs are still ozone-depleting substances used in air conditioning and refrigeration systems.
6. Nitrous Oxide (N2O): This gas, emitted from agricultural and industrial activities, can also contribute to ozone depletion in the stratosphere.
Now, let’s look at a few natural ozone depletion causes that you can add in the ozone layer depletion essay.
1. Solar Variability: Changes in solar radiation and sunspot activity can influence the amount of ultraviolet radiation reaching the Earth’s atmosphere. However, these variations are relatively small and have a limited effect on ozone depletion.
2. Volcanic Eruptions: Large volcanic eruptions can strike sulphur compounds into the stratosphere. These sulfuric acid aerosols can eventually lead to localised ozone depletion, but the effect is generally short-lived and limited in scope.
3. Cosmic Rays: High-energy cosmic rays from space can interact with atmospheric molecules, thus producing reactive nitrogen oxides that can influence ozone chemistry. The contribution of cosmic rays to ozone layer depletion is considered to be minimal.
4. Stratospheric Changes: Natural processes within the stratosphere, such as the dynamics of stratospheric circulation patterns and temperature fluctuations, can influence ozone levels to an extent. However, these processes are interconnected with human-produced ozone depletion and are not independent of natural causes.
Consequences of Ozone Layer Depletion
The next important segment in the Ozone layer depletion essay is the possible consequences of layer depletion. Have a look!
Ozone layer depletion has significant consequences for both the environment and human health. Some of the key consequences include:
1. Increased Ultraviolet (UV) Radiation: Depletion of the ozone layer allows more UV radiation to reach the Earth’s surface. This increased UV radiation can have several detrimental effects:
- Skin Cancer: Higher UV levels are linked to an increased risk of skin cancer, including melanoma.
- Cataracts: UV radiation exposure can contribute to the development of eye cataracts.
- Weakened Immune System: UV radiation can suppress the immune system, making individuals more susceptible to diseases.
2. Damage to Marine Ecosystems: UV radiation can penetrate the ocean’s surface and harm marine ecosystems. It can affect phytoplankton, which form the base of the marine food web, and disrupt aquatic ecosystems.
3. Harm to Terrestrial Ecosystems: Increased UV radiation can damage terrestrial plants, reducing crop yields and affecting natural vegetation. This can impact food production and ecosystem stability.
4. Disruption of Aquatic Food Chains: UV radiation can harm aquatic organisms, including fish larvae, zooplankton, and amphibians, disrupting aquatic food chains and ecosystems.
5. Ozone Holes: In some places, especially over Antarctica, severe ozone depletion forms “ozone holes.” These holes can persist for extended periods, allowing intense UV radiation to reach the surface and causing acute environmental and health risks.
6. Climate Change Issues: Ozone depletion can impact atmospheric circulation patterns and climate. It can also indirectly contribute to global warming because some ozone-depleting substances, like CFCs, are also potent greenhouse gases.
7. Agricultural Impacts degradation: Increased UV radiation can harm crops and reduce agricultural yields, leading to potential food shortages and economic losses.
Global Initiatives to Fight Ozone Layer Depletion
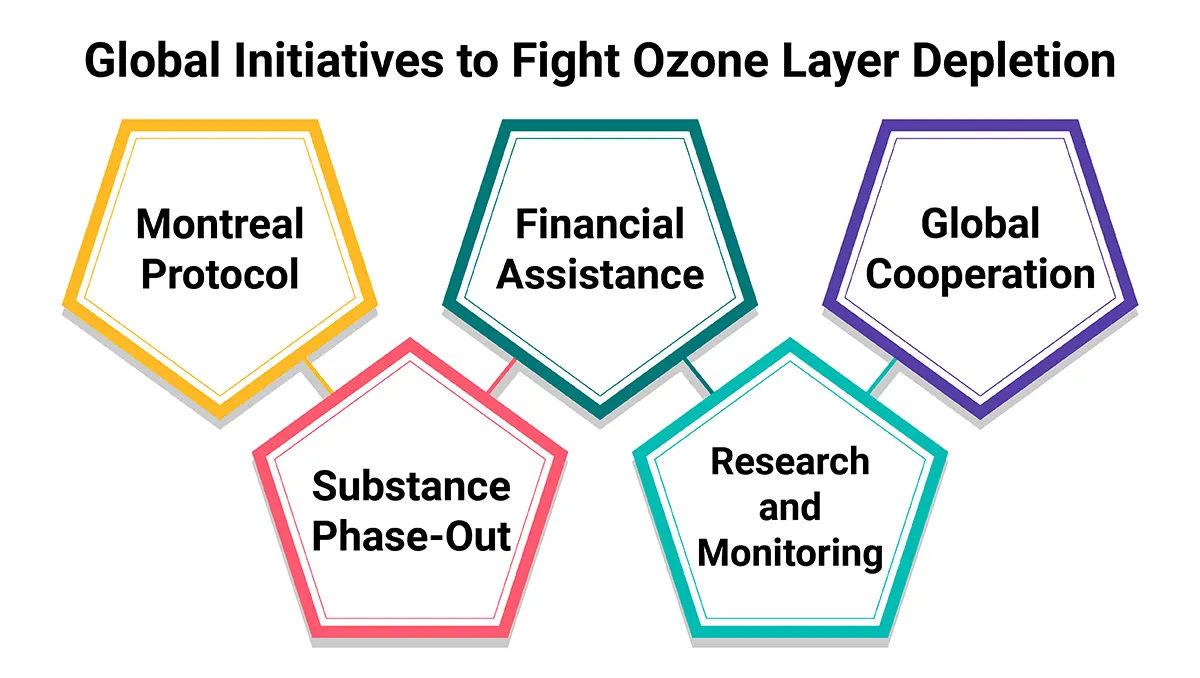
The depletion of the ozone layer is caused by various manmade and natural factors, and here’s how global initiatives are taken to battle the problem.
Global initiatives to combat ozone layer depletion in your ozone layer depletion essay. The most significant initiative is the Montreal Protocol on Substances that Deplete the Ozone Layer, which was adopted in 1987 and has been amended several times to strengthen its provisions. Here are some key elements of these global initiatives:
1. Montreal Protocol: The Montreal Protocol is an international treaty aimed at phasing out ozone-depleting substances (ODS) production and consumption. It sets specific schedules and targets for the phase-out of these substances. Almost all countries in the world have ratified the Protocol.
2. Substance Phase-Out: The Protocol initially focused on the phase-out of chlorofluorocarbons (CFCs), halons, carbon tetrachloride, and other major ODS. Subsequent amendments added additional substances like hydrochlorofluorocarbons (HCFCs) and methyl chloroform.
3. Financial Assistance: To help developing countries transition from ODS, the Protocol established the Multilateral Fund to implement the Montreal Protocol. This fund provides financial and technical assistance to developing countries to support their efforts in phasing out ODS.
4. Research and Monitoring: The Protocol encourages research and monitoring activities related to ozone depletion and the identification of ozone-friendly alternative substances and technologies.
5. Global Cooperation: The success of these initiatives is a testament to global cooperation in addressing environmental challenges. Countries, industries, and environmental organisations have worked together to implement the Protocol’s provisions.
These global initiatives to fight ozone layer depletion have been widely regarded as a success story in international environmental cooperation.
Future Predictions and Steps Forward
In the next segment of the ozone layer depletion essay, write about controlling the ozone layer depletion and the key steps it involves the key steps. It’s a global concern now, as we are solely responsible for protecting Mother Earth and her dear people from the harsh consequences of the depletion.
With new technological innovations and research coming up, new methods can be implemented to resist the depletion. However, humans play an integral role in controlling harmful chemicals directly linked with affecting the ozone layer.
Role of Man in Protecting Ozone Layer
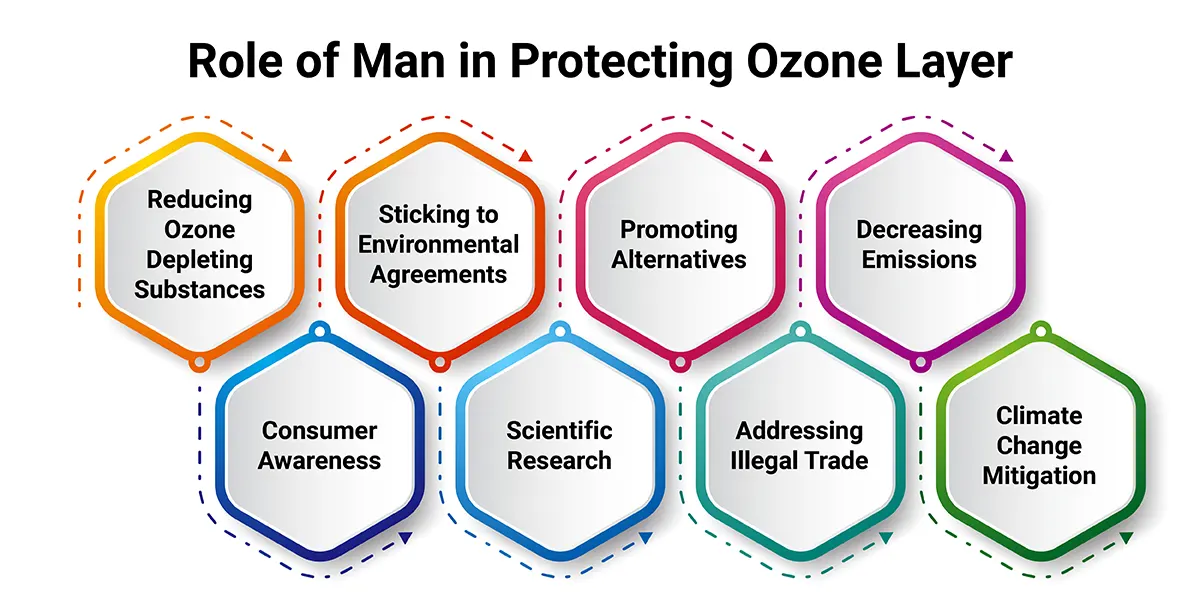
We have discussed several issues in this ozone layer depletion essay, and now let’s look at how humans can contribute towards protecting the ozone layer and safeguarding our mother Earth.
Human actions play a crucial role in protecting the ozone layer. The primary ways in which we can contribute to ozone layer protection include:
1. Reducing Ozone-Depleting Substances: The most significant human contribution is reducing ozone-depleting substances, such as chlorofluorocarbons (CFCs), halons, and carbon tetrachloride. This is achieved through international agreements like the Montreal Protocol, which phases out the production and consumption of these substances.
2. Sticking to Environmental Agreements: Governments and industries should comply with international agreements and regulations, like the Montreal Protocol, to control the production and use of ozone-depleting chemicals. Enforcement of these agreements is essential to limit the release of harmful substances into the atmosphere.
3. Promoting Alternatives: Encouraging the development and use of ozone-friendly alternatives to ozone-depleting substances in various applications, such as air conditioning, refrigeration and aerosol propellants.
4. Decreasing Emissions: Preventing leaks and emissions of ozone-depleting chemicals during manufacturing, transportation, and disposal of equipment containing these substances.
5. Consumer Awareness: Educating the public about the importance of protecting the ozone layer and encouraging responsible consumption and disposal of products containing ozone-depleting substances.
6. Scientific Research: Supporting scientific research to understand better the ozone layer, its depletion, and recovery processes, which can inform policy-making and mitigation efforts.
7. Addressing Illegal Trade: Combating the illegal trade of ozone-depleting substances can undermine international efforts to protect the ozone layer.
8. Climate Change Mitigation: Recognising the link between ozone layer protection and climate change mitigation, as some ozone-depleting substances are also potent greenhouse gases. Addressing both issues together can yield significant environmental benefits.
Human actions, both at the individual and societal levels, are critical in preserving and restoring the ozone layer. International cooperation and continued vigilance are a must to ensure its long-term protection.
Debunking the Myths on Ozone Layer Depletion
There’s no denying that tons of myths revolving around Ozone Layer depletion need to be stopped. Some of the common myths are:
- The Antarctic Ozone “hole” was present all along; it was discovered in the 1970s because that’s when satellite measurements began.
- Volcanoes and other natural causes contribute much more chlorine to the ozone layer than CFC.
- CFCs are unable to reach the stratosphere because they’re heavier than air.
All these misconceptions revolve around Ozone, but recent study findings have helped people gather relevant data about it.
Wrapping Up
With that, we conclude the ozone layer depletion essay. While progress has been made to control the depletion, complete restoration of the ozone layer will take several more decades. Continued adherence to international agreements like the Montreal Protocol and ongoing research and monitoring is crucial to prevent further depletion and ultimately let the ozone layer heal.
So, while we can’t stop it overnight, we can stick to the environmental guidelines for a better tomorrow. Global solid efforts have effectively mitigated ozone layer depletion and offer hope for its eventual recovery.
Also Read: World Ozone Day
FAQs on Ozone Layer Depletion Essay
Q1. What measures can you take to reduce ozone layer depletion at home?
Most chemical-based clinical products include chlorine and bromine that finds a way to reach the atmosphere. Switching to more natural cleaning products can save the ozone layer.
Q2. What can the government do to minimise ozone layer depletion?
The government can ban the use of nitrous oxide or take action against its usage to minimise ozone layer depletion. Mass awareness should be spread too to minimise the usage.
Q3. What are the greenhouse gases that cause ozone layer depletion?
Carbon dioxide, CFCs, methane, nitrous oxide.
Q4. How many oxygen atoms make up the ozone gas?
Three oxygen atoms.

