NCERT Solutions for Class 12 Chemistry Chapter 7 - The p-Block Elements
NCERT Solutions for Class 12 Chemistry Chapter 7 Free PDF Download
Please Click on Free PDF Download link to Download the NCERT Solutions for Class 12 Chemistry Chapter 7 The p-Block Elements
1. Discuss the general characteristics of Group 15 elements with reference to their electronic configuration, oxidation state, atomic size, ionisation enthalpy and electronegativity.
Ans. General characteristics of group 15 elements.
(i) Electronic configuration: Valence shell configuration of elements of group 15 is ns2 np3. s-orbital of these elements is completely filled and p-orbital is half-filled, making their electronic configuration extra stable.
| Minerals | Ores |
| Nitrogen | (7N) = [He] 2s2 2p3 |
| Phosphorus | (15P) = [Ne] 3s2 3p3 |
| Arsenic | (33As) = [Ar] 3d10 4s2 4p3 |
| Antimony | (51Sb) = [Kr] 4d10 5s2 5p3 |
| Bismuth | (83Bi) = [Xe] 4f14 5d10 6s26p3 |
(ii) Oxidation state: Common oxidation states exhibited by elements of this group are –3, +3 and +5. The tendency to exhibit –3 oxidation state decreases down the group due to increase in size and metallic character. Bi (last member of the group) hardly forms any compound of –3 oxidation state.
The stability of +5 oxidation state decreases down the group, whereas that of +3 oxidation state increases down the group (due to inert pair effect). N reacts with oxygen showing +1, +2, +3, +4 and +5 oxidations states. P shows +1 and +4 oxidation states in some oxo-acids.
(iii) Atomic size: Covalent and ionic radii (in a particular state) increase down the group. From N to P, covalent radius increases considerably. From As to Bi, there is only a small increase in covalent radius. The reason is the presence of completely filled d and/or f orbitals in heavier members.
(iv) Ionisation enthalpy: Ionisation enthalpy decreases down the group due to gradual increase in atomic size. I.E., of group 15 elements is greater than that of group 14 elements and group 16 elements in the corresponding periods. The order of successive ionisation enthalpies as expected is
∆iH1 < ∆iH2 < ∆iH3
(v) Electronegativity: The electronegativity value decreases down the group with increasing atomic size.
2. Why does the reactivity of nitrogen different from phosphorus?
Ans. Nitrogen exists as a diatomic molecule (N ≡ N). Due to the presence of a triple bond between the two N-atoms, the bond dissociation energy is large (941.4 kJ mol–1). This, nitrogen is inert and unreactive in its elemental state. In contrast, white or yellow phosphorus exists as a tetraatomic molecule (P4). Since, the P—P single bond is much weaker (213 kJ mol–1) than N ≡ N triple bond (941.4 kJ mol–1) therefore, phosphorus is much more reactive than nitrogen.
3. Discuss the trends in chemical reactivity of group 15 elements.
Ans. Trends in chemical reactivity of group 15 elements.
(i) Oxidation state: Same as in Question no. 1.
(ii) Reactivity towards hydrogen: These elements form covalent hydrides with the formula EH3.
[where, E = N, P , As, Sb]
(a) Thermal stability of hydrides decreases down the group.
(b) Reducing character of hydrides increases down the group.
(c) Basic character of hydrides decreases down the group.
(d) Boiling point of NH3 is greater than PH3 due to H-bonding. Boiling points of other hydrides increase from PH3 onwards.
Lighter elements like nitrogen also form hydrides of the formula E2H4. e.g., N2H4
(iii) Reactivity towards halogens: All group 15 elements i.e., N, P, As, Sb, Bi form
(a) Halides of general formula EX3. Except NBr3 and NI3, all are stable with pyramidal shape. These get hydrolysed by water.
(b) Halides of general formula EX5. These are formed by P, As and Sb but not by N. These halides are trigonal bipyramidal with sp3d hybridisation.
(iv) Reactivity towards oxygen: These elements form oxides of the formula E2O3 and E2O5. However, nitrogen because of its tendency to form pπ–pπ multiple bonds, form a range of oxides having oxidation states +1 to +5 (e.g., N2O, NO, N2O3, NO2, N2O4, N2O5) E2O5 are more acidic than E2O3. Acidic character of oxides decreases down the group.
4. Why does NH3 form hydrogen bond but PH3 does not?
Ans. Nitrogen has an electronegativity value 3.0, which is much higher than that of PH3 (2.1). As a result, N – H bond is quite polar and hence NH3 undergoes intermolecular PH3 – bonding.

5. How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions involved.
Ans. (i) Laboratory preparation of nitrogen gas:
In the laboratory, dinitrogen is prepared by treating an aqueous solution of ammonium chloride with sodium nitrite.
$$\text{NH}_{4}\text{Cl}(aq)+\text{NaNO}_{2}(aq)\xrightarrow{}\text{N}_{2}(g)+2\text{H}_{2}\text{O(l)}+\text{NaCl}(aq)$$
Small amounts of NO and HNO3 are also formed as impurities, which can be removed by passing the gas through aqueous sulphuric acid containing potassium dichromate.
(ii) Dinitrogen can also be prepared by heating ammonium dichromate
$$\text{(NH}_{4})\text{Cr}_{2}\text{O}_{7}\text{(s)}\xrightarrow{\Delta}\text{N}_{2}(g)+4\text{H}_2\text{O(l)}+\text{Cr}_{2}\text{O}_{3}$$
(iii) Pure nitrogen is obtained by thermal decomposition of sodium or barium azide.
$$\text{Ba(N}_{3})_{2}\xrightarrow[\text{Decomposition}]{\text{Thermal}}\text{Ba + 3N}_{2}$$
6. How is ammonia manufactured industrially?
Ans. Ammonia is manufactured industrially by Haber’s process from nitrogen and hydrogen.
$$\text{N}_{2}(g)+3\text{H}_{2}(g)\xrightleftharpoons[\Delta_f\text{H}\degree=-46.1\space \text{kJ mol}^{\normalsize-1}]{\text{Fe}_{2}\text{O}_{3}/\text{K}_{2}\text{O \space and Al}_{2}\text{O}_{3}, 700k,200\space\text{atm}}\text{2NH}_{3}(g)$$
According to Le-Chatelier’s principle, high pressure would favour the production of ammonia. Optimum conditions for production of NH3 are:
(i) Temperature ~ 700 K
(ii) Pressure – 200 × 105 Pa (or 200 atm)
(iii) Catalyst – Fe2O3
(iv) Promotor – K2O and Al2O3
Note: Earlier Fe was used as catalyst with molybdenum.

7. Illustrate how copper metal can give different products on reaction with HNO3.
Ans. (i) Copper on heating with dilute HNO3, produces copper nitrate and nitric oxide.
$$\underset{\text{(dilute)}}{3\text{3Cu + 8HNO}_{3}}\xrightarrow{\Delta}3\text{3Cu(NO}_{3})_{2}+4\text{H}_{2}\text{O}+\text{2NO}$$
(ii) On heating with concentrate HNO3, copper produces copper nitrate with the evolution of nitrogen dioxide.
$$\underset{(\text{conc})}{\text{Cu + 4HNO}_{3}}\xrightarrow{\Delta}\text{Cu (NO}_{3})_{2}+\text{2H}_{2}\text{O}+2\text{NO}_{2}$$
8. Give the resonating structures of NO2 and N2O5.
Ans. NO2 :

9. The HNH angle value is higher than HPH, H AsH and HSbH angles. Why?
(Hint: Can be explained on the basis of sp3 hybridisation in NH3 and only s-p bonding , between hydrogen and other elements of the group).
Ans. In all these cases, the central atom is sp3 hybridised. Three of the four sp3 orbitals form three σ-bonds, while the fourth contains the lone pair of electrons. On moving down from N to Sb, the electronegativity of the central atom goes on decreasing. As a result of this, bond pairs of electrons lie away and away from the central atom. This is because of the force of repulsion between the adjacent bond pairs goes on decreasing and the bond angles keep on decreasing from NH3 to SbH3. Thus, bond angles are in the order:
HNH > HPH > HAsH > HSbH
(107.8°) (93.6°) (91.8°) (91.3°)
10. Why does R3P = O exist but R3N = O does not (R is an alkyl group)?
Ans. Nitrogen cannot form pπ – dπ multiple bonds, due to the absence of d-orbitals. As a result, the covalency of nitrogen cannot be expanded beyond 4. However, in R3N = O, the covalency of nitrogen is 5. Hence, R3N = O does not exist. On the other hand, phosphorus forms pπ – dπ multiple bonds, due to the presence of d-orbitals. As a result, P can expands its covalency beyond 4 and form R3P = O, in which its covalency is 5.
11. Explain why NH3 is basic while BiH3 is only feebly basic.
Ans. In both NH3 and BiH3, N and Bi have a lone pair of electrons on the central atom and hence should behave as Lewis bases. But NH3 is much more basic than BiH3. Since the atomic size of N is much smaller than that of Bi, therefore, electron density on N-atom is much higher than that on Bi-atom. Thus, the tendency of N in NH3 to donate its lone pair of electrons is much more in comparison to tendency of Bi in BiH3. Hence, NH3 is more basic than BiH3.
12. Nitrogen exists as diatomic molecule and phosphorus as P4. Why?
Ans. Nitrogen exists as a diatomic molecule having a triple bond between the two N-atoms, This is due its small size that it forms pπ–pπ multiple bonds with itself and with carbon/oxygen as well. On the other hand, phosphorus due to its larger size does not form multiple pπ–pπ bonds with itself. It prefers to form P – P single bonds and hence it exists as tetrahedral P4 molecule.
13. Write main differences between the properties of white phosphorus and red phosphorus.
Ans.
| Property | White Phosphorus | Red Phosphorus | |
| (1) | State | Translucent | Brittle, substance |
| (2) | Colour | White gets yellowish on exposure to light | Red |
| (3) | Odour | Garlic like odour | Odourless |
| (4) | Hardness | Soft like wax and can be cut by knife | Hard |
| (5) | Poisonous nature | Poisonous | Non-poisonous |
| (6) | Solubility | Soluble in CS2 | Insoluble in CS2 |
| (7) | Chemiluminescence | Glows in dark | Dose not glow in dark |
| (8) | Density | 1.8 | 2.1 |
| (9) | Reactivity | Very reactive | Less reactive |
| (10) | Action of oxygen | Bums with greenish glow to from P4O10 | Combines with O2 only on heating to form P4O10 |
14. Why does nitrogen show catenation properties less than phosphorus?
Ans. Catenation depends on the strength of interatomic bond. The N–N bond strength (159 kJ/mol) is much weaker than P–P bond strength (213 kJ/mol). Therefore, catenation property of nitrogen is less than that of phosphorus.
15. Give the disproportionation reaction of H3 PO3.
Ans. On heating, H3 PO4 undergoes self oxidation reduction, i.e., disproportionation to form PH3.
$$\underset{\text{Phosphorus acid}}{4\text{H}_{3}\text{PO}^{\normalsize+3}_{3}}\xrightarrow{\Delta}\underset{\text{Phosphine}}{\text{PH}_{3}^{\normalsize-3}}+\underset{\text{Orthophosphoricacid}}{3\text{H}_{3}\text{PO}^{\normalsize+5}_{4}}$$
16. Can PCl5 act as an oxidising as well as a reducing agent? Justify.
Ans. The oxidation state of P in PCl5 is +5. Since P has five electrons in its valence shell, therefore, it cannot donate electron and cannot increase its oxidation state beyond +5, Thus, PCl5 cannot act as a reducing agent. It can act as oxidizing agent by itself undergoing reduction.
$$\text{P}^{\normalsize+5}\text{Cl}_{5}+\text{H}_{2}^{0}\xrightarrow{}\text{P}^{\normalsize+3}\text{Cl}_{3}+2\text{H}^{\normalsize+1}\text{Cl}\\2\text{Ag}^{0}+\text{P}^{\normalsize+5}\text{Cl}_{5}\xrightarrow{}2\text{Ag}^{\normalsize+1}\text{Cl}+\text{P}^{\normalsize+3}\text{Cl}_{3}$$
17. Justify the placement of O, S, Se, Te and Po in the same group of the periodic table in terms of electronic configuration, oxidation state and hydride formation.
Ans. (i) Electronic configuration:
O (At. no. = 8) = [He] 2s2 2p4
S (At. no. = 16) = [Ne] 3s2 3p4
Se (At. no. = 34) = [Ar] 3d10 4s2 4p4
Te (At. no. = 52) = [Kr] 4d10 5s2 5p4,
Po (At. no. = 84) = [Xe] 4f 14 5d10 6s2 6p4,
Thus, all these elements have the same ns2 np4 (n = 2 to 6) valence shell electronic configuration, hence are justified to be placed in group 16 of the Periodic Table.
(ii) Oxidation state: Two more electrons are needed to acquire the nearest noble gas configuration. Thus, the minimum oxidation state of these elements should be –2. O and to some extent S show –2 oxidation state. Other element being more electropositive than O and S, do not show negative oxidation state. As these contain six electrons, thus, maximum oxidation state shown by them is +6. Other oxidation state shown by them are +2 and +4. O do not show +4 and +6 oxidation state, due to the absence of d-orbitals. Thus, on the basis of maximum and minimum oxidation states, these elements are justified to be placed in the same group 16 of the periodic table.
(iii) Hydride formation: All these elements share two of their valence electrons with 1 s-orbital of hydrogen to form hydrides of the general formula EH2, i.e., H2O, H2S, H2Se, H2Te and H2Po. Thus, on the basis of hydride formation, these elements are justified to be placed in the same group 16 of the Periodic Table.
18. Why is dioxygen a gas but sulphur a solid?
Ans. The O—O bond is weaker than the S—S bond due to greater inter-electronic repulsions in small sized oxygen atom moreover being smaller and highly electronegative oxygen forms pπ–pπ multiple bonds. So, it exists as O2 molecules which are held together by weak Van der Waals’ force. Thus, oxygen exists as a gas at room temperature. sulphur has less tendency to form pπ–pπ multiple bonds. Moreover, it has large atomic size, less electronegativity and forms strong S—S single bonds, that’s why it shows more catenation property and exists as S8 molecules having puckered ring structure. Hence, sulphur is a solid at room temperature.
19. Knowing the electron gain enthalpy values of O → O– and O → O2– as –141 and 702 kJ mol–1 respectively, how can you account for the formation of a large number of oxides having O2– species and not O–?
Ans. Let us consider the reaction of oxygen with monopositive metal, we can have two compounds. MO (O in –1 state) and M2O (O in –2 state). The energy required for formation of O–2 is compensated by increased coulombic attraction between M+ and O–2. Coulombic force of attraction, FA is proportional to product of charges on ions i.e.
$$\text{F}_{\text{A}}∝\frac{q_1q_2}{r^{2}}$$
Where, q1 and q2 are charges on ions and r is distance between ions. Same logic can be applied if metal is dispositive.
20. Which aerosols deplete ozone?
Ans. Aerosols like chlorofluorocarbons (CFC’s), i.e., freon (CCl2F2), depletes the ozone layer by supplying Cl* free radicals which convert O3 to O2.
$$\underset{\text{Freon}}{\text{CCl}_{2}\text{F}_{2}(g)}\xrightarrow{hv}\text{Cl(g)}+.\text{CClF}_{2}(g)\\\text{Cl(g) + O}_{3}(g)\xrightarrow{}\text{ClO}.(g)+\text{O}_{2}\text{(g)}\\\text{ClO.(g)}+.\text{O(g)}\xrightarrow{}.\text{Cl(g)+\text{O}}_{2}(g)$$
21. Describe the manufacture of H2SO4 by contact process?
Ans. Manufacture of sulphuric acid (contact process)
It involves following steps:
(i) Production of sulphur dioxide: It is carried out by burning powdered sulphur or roasting of sulphur rich ores.
$$\text{S}_{8}+8\text{O}_{2}\xrightarrow{}8\text{SO}_{2}\\4\text{FeS}_{2}+11\text{O}_{2}\xrightarrow{}2\text{Fe}_{2}\text{O}_{3}+8\text{SO}_{2}$$
(ii) Oxidation of sulphur dioxide to sulphur trioxide
$$2\text{SO}_{2}(g)+\text{O}_{2}(g)\xrightleftharpoons[1.5-2\text{atom}]{400-450\degree\text{C}}2\text{SO}_{3}(g)+\text{Heat}$$
This is the major step to the contact process.
For better yield of SO3, conditions required are :
Temperature = 400 – 450°C;
Pressure = 1.5 to 2 atm;
Catalyst = Pt asbestos or vanadium pentoxide V2O5.
(iii) Conversion of SO3 into H2SO4: SO3 is absorbed in conc. H2SO4 to produce oleum or pyrosulphuric acid.
$$\underset{\text{Conc.}}{\text{SO}_{3}+\text{H}_{2}\text{SO}_{4}}\xrightarrow{}\underset{\text{Oleum}}{\text{H}_{2}\text{S}_{2}\text{O}_{7}}$$
SO3 is not absorbed in water as the reaction is highly exothermic and a mist of acid particles is formed. Sometimes it becomes difficult to handle the operation.
(iv) Dilution of oleum and formation of H2SO4: Oleum is diluted with calculated quantity of water to obtain H2SO4 in required concentration.
$$\underset{\text{Oleum}}{\text{H}_{2}\text{SO}_{4}}\xrightarrow{}\underset{\text{Oleum}}{\text{H}_{2}\text{S}_{2}\text{O}_{7}}$$
$$\underset{\text{Oleum}}{\text{H}_{2}\text{S}_{2}\text{O}_{7}}+\underset{\text{Water}}{\text{H}_{2}\text{O}}\xrightarrow{}\underset{\text{Sulphuric acid}}{2\text{H}_{2}\text{SO}_{4}}$$

22. How is SO2 an air pollutant?
Ans. (i) SO2 dissolves in moisture present in air to form H2SO4 which damages building materials especially marble (acid – rain).
$$\text{CaCO}_{3}+\text{H}_{2}\text{SO}_{3}\xrightarrow{}\text{CaSO}_{3}+\text{H}_{2}\text{O}+\text{CO}_{2}$$
(ii) It corrodes metals like Fe and steel. It also brings about fading and deterioration of fabrics, leather, paper, etc., and affecting the colour of paints.
(iii) Even in low concentration (= 0.03 ppm), it has damaging effect on the plants. If exposed for a long time, i.e., a few days or weeks, it slows down the formation of chlorophyll i.e., loss of green colour. This is called chlorosis.
(iv) It is strongly irritating to the respiratory track. It cause throat and eye irritation, resulting into cough, tears and redness in eyes. It also cause breathlessness and effects larynx i.e., voice box.
23. Why are halogens strong oxidising agents?
Ans. Halogens have a strong tendency to accept electrons and get reduced. This is due to low bond dissociation enthalpy, high electronegativity and large electron gain enthalpy of halogens.
$$\text{X}_{2}+2e\xrightarrow{}2\text{X}^{\normalsize-}$$
As a result, halogens are strong oxidising agents. However, their oxidising power decreases from F2 to I2.
24. Explain why fluorine forms only one oxoacid, HOF.
Ans. Cl, Br and I form four series of oxo acids of general formula HOX, HOXO, HOXO2, and HOXO3. In these oxo-acids, the oxidation states of halogens are +1, +3, +5, and +7 respectively. However, due to high electronegativity, small size and absence of d-orbitals, F does not form oxo-acids with +3, +5 and +7, oxidation states. It just forms one oxo-acid (HOF).
25. Explain why inspite of nearly the same electronegativity, nitrogen forms hydrogen bonding while chlorine does not.
Ans. O and Cl have different atomic size (covalent radii), (O = 66 pm, Cl = 99 pm), although have the nearly same electrogatively. As a result, electron density per unit volume of oxygen atom is much higher than that of Cl atom. Hence, oxygen form hydrogen bonds and Cl cannot.
26. Write two uses of ClO2.
Ans. (i) ClO2 is an excellent bleaching agent. It is 30 times stronger bleaching agent than the Cl2. It is used as a bleaching agent for paper pulp in paper industry and in textile industry.
(ii) ClO2 is also a powerful oxidising agent and chlorinating agent. It acts as a germicide for disinfecting water. It is used for purifying drinking water.
27. Why are halogens coloured?
Ans. The halogens are coloured because their molecules absorb light in the visible region. As a result, of which their electrons get excited to higher energy levels while the remaining light is transmitted. The colour of halogens is the colour of this transmitted light.
28. Write the reactions of F2 and Cl2 with water.
Ans. F2 is a strong oxidant , If oxidises H2O to O2 or O3.
$$2\text{F}_{2}\text{(g)}+2\text{H}_{2}\text{O}(l)\xrightarrow{}4\text{H}^{\normalsize+}(aq)+4\text{F}^{\normalsize-}(aq)+\text{O}_{2}(g)\\3\text{F}_{2}(g)+3\text{H}_{2}\text{O}(l)\xrightarrow{}6\text{H}^{\normalsize+}(aq)+6\text{F}^{\normalsize-}(aq)+\text{O}_{3}(g)\\\text{On the other hand, Cl}_2 \text{reacts with H}_2\text{O to form hydrocloric acid and hypochlorous acid.}\\\text{Cl}_{2}(g)+ \text{H}_{2}\text{O(l)}\xrightarrow{}\text{HCl(aq) + HOCl(aq)}$$
29. How can you prepare Cl2 from HCl and HCl from CI2? Write reactions only.
Ans. HCl can be oxidised to Cl2 using various oxidising agents such as MnO2, KMnO4, K2Cr2O7 etc.
$$\underset{\text{Oxidising agent}}{\text{MnO}_{2}+4\text{HCl}}\xrightarrow{}\text{MnCl}_{2}+\text{Cl}_{2}+2\text{H}_{2}\text{O}\\\text{Cl}_2 \text{can be reduced to HCl using H}_2 \text{in the presence of diffused Sunlight.}\\\text{H}_{2}+\text{Cl}_{2}\xrightarrow{\text{Diffused sunlight}}2\text{HCl}$$
30. What inspired N. Bartlett for carrying out reaction between Xe and PtF6?
Ans. N. Bartlett observed that PtF6 reacts with O2 to give an compound O2+ [PtF6]–.
Since the first ionisation enthalpy of Xe (1170 kJ mol–1) is fairly close to that of 02 molecule (1175 kJ mol–1), he thought that PtF6 should also oxidise Xe to Xe+. This inspired Bartlett to carryout the reaction between Xe and PtF6. When PtF6 and Xe were made to react, a rapid reaction took place and a red solid, Xe+ [PtF6]– was obtained.
$$\text{Xe + PtF}_{6}\xrightarrow{\text{273 \text{K}}}\text{Xe}^{+}[\text{PtF}_{6}]^{\normalsize-}$$
31. What are the oxidation states of phosphorus in the following:
(i) H3PO3
(ii) PCl3
(iii) Ca3P2
(iv) Na3PO4
(v) POF3
Ans. (i) H3PO3
3(+1) + x + 3(–2) = 0
∴ x = +3
(ii) PCl3
x + 3(–1) = 0
x = +3
(iii) Ca3P2
3(+2) + 2x = 0
x = –3
(iv) Na3PO4
3(+1) + x + 4(–2) = 0
x = +5
(v) POF3
x + 1(–2) + 3(–1) = 0
x = +5
32. Write balanced equations for the following:
(i) NaCl is heated with sulphuric acid in the presence of MnO2
(ii) Chlorine gas is passed into a solution of NaI in water.
$$\textbf{Ans}\space\text{(i) NaCl +H}_{2}\text{SO}_{4}\xrightarrow{}\text{NaHSO}_{4}+\text{HCl×4}\\\text{4HCl + MnO}_{2}\xrightarrow{}\text{MnCl}_{2}+\text{Cl}_{2}+2\text{H}_{2}\text{O}\\\text{(ii) Cl}_{2}(g)+ 2\text{NaI}(aq)\xrightarrow{}2\text{NaCl}(aq)+\text{I}_{2}(s)$$
33. How are xenon fluorides XeF2, XeF4 and XeF6 obtained?
Ans. XeF2, XeF4 and XeF6 are obtained by direct reaction between Xe and F2 as follows:
$$\underset{\text{Excess}}{\text{Xe(g)+F}_{2}(g)}\xrightarrow[\text{Ni tube}]{673\text{K}, 1\text{bar}}\text{XeF}_{2}(s)\\\underset{(\text{In 1:5 ratio})}{Xe(g) + 2\text{F}_{2}}(g)\xrightarrow{\text{873 K, 7 bar}}\text{XeF}_{4}(s)\\\underset{\text{(In 1:20 ratio)}}{\text{Xe(g) + 3F}_{2}(g)}\xrightarrow{\text{573 K, 60-70 \text{bar}}}\text{XeF}_{6}(s)$$
34. With what neutral molecules is ClO– isoelectronic? Is this molecule Lewis base?
Ans. ClO– has (17 + 8 + 1) = 26 electrons. It is isoelectronic with two neutral molecules.
Oxygen difluoride (OF2) : 8 + 18 = 26 electrons
Chlorine fluoride (ClF) : 17 + 9 = 26 electrons
Out of these, ClF can act as Lewis base. The atom chlorine has three lone electron pairs which it donates to form compounds like ClF3, ClF5 and ClF7.
35. How are XeO3 and XeOF4 prepared?
Ans. XeO3 is prepared by complete hydrolysis:
$$\text{6XeF}_{4}+12\text{H}_{2}\text{O}\xrightarrow{\text{Hydrolysis}}\space 4\text{Xe + 2XeO}_{3}+24\text{HF + 3\text{O}}_{2}\\\text{XeF}_{6} + 3\text{H}_{2}\text{O}\xrightarrow{\text{Hydrolysis}}\text{XeO}_{3} + 6\text{HF}\\\text{XeO}_4 \text{F} _4 \text{is prepared by partial hydrolysis:}\\\text{XeF}_{6}+\text{H}_{2}\text{O}\xrightarrow{\text{Hydrolysis}}\text{XeOF}_{4}+2\text{HF}$$
36. Arrange the following in the order of property indicated for each set:
(i) F2 , Cl2 , Br2< > , I2 – increasing bond dissociation enthalpy.
(ii) HF, HCI, HBr, HI – increasing acid strength.
(iii) NH3, PH3, AsH3, SbH3, BiH3 – increasing base strength.
Ans. (i) Bond dissociation enthalpy decreases as the bond distance increases from F2 to I2 due to increase in the size of the atom, on moving from F to I.
F—F bond dissociation enthalpy is smaller then the Cl—Cl and even smaller than Br—Br. This is because F atom is very small and have large electron-electron repulsion among the lone pairs of electrons in F2 molecule where they are much closer to each other than in case of Cl2. The increasing order of bond dissociation enthalpy is I2 < F2 < Br2 < Cl2.
(ii) Acid strength of HF, HCl, HBr and HI depends upon their bond dissociation enthalpies. Since the bond dissociation enthalpy of H—X bond decreases from H—F to H—l as the size of atom increases from F to I.
Thus, the acid strength order is HF < HCI < HBr < HI
The weak acidic strength of HF is also due to H-bonding due to which release of H+ becomes difficult.
(iii) NH3, PH3, ASH3, SbH3 and BiH3 behaves as Lewis bases due to the presence of lone pair of electrons on the central atom. As we move from N to Bi, size of atom increases. Electron density on central atom decreases and hence the basic strength decreases from NH3 to BiH3. Thus, basic strength order is
BiH3 < SbH3 < AsH3 < PH3 < NH3
37. Which one of the following does not exist ?
(i) XeOF4
(ii) NeF2
(iii) XeF4
(iv) XeF6
Ans. The sum of the first two ionisation enthalpies of Ne are much higher than those of Xe. Hence, F2 can oxidise Xe to Xe2+ but can not oxidise Ne to Ne2+. Therefore, NeF2 does not exist.
38. Give the formula and describe the structure of a noble gas species which is isostructural with:
(i) ICl4–
(ii) IBr2–
(iii) BrO3–
Ans.
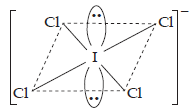
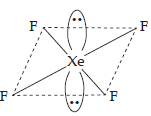
| Given compound | Isostructural noble gas species | |||
| (1) | (a) | ICl4– | (a) | XeF4 |
| (b) | Square planar geometry. | (b) | Square planar geometry. | |
| (c) | Valence electrons = 36. | (c) | Valence electrons = 36. | |
| (d) | Four bonded pairs and 2 lone pairs, of electrons with I. | (d) | Four bonded pairs and two lone pairs with Xe. | |
| (e) | Structure | (e) | Structure | |
 |
 |
|||
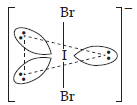
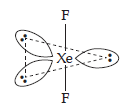
| (2) | (a) | IBr2– | (a) | XeF2 |
| (b) | Linear molecule or trigonal bipyramidal. | (b) | Linear molecules or trigonal bipyramidal. | |
| (c) | Valence electrons = 22 | (c) | Valence electrons = 22. | |
| (d) | 3 lone pairs and 2 bonded pairs. | (d) | 3 lone pairs and 2 bonded pairs. | |
| (e) | Structure | (e) | Structure | |
 |
 |
|||
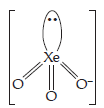
| (3) | (a) | BrO3– | (a) | XeO3 |
| (b) | Pyramidal shape. | (b) | Pyramidal shape. | |
| (c) | Valence electrons = 26. | (c) | Valence electrons = 26. | |
| (d) | 3 bonded pairs and 1 lone pair. | (d) | 3 bonded pairs and 1 lone pair. | |
| (e) | Structure | (e) | Structure | |
 |
(e) | 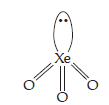 |
39. Why do noble gases have comparatively large atomic size?
Ans. Atomic radii of noble gases are largest in their respective periods. Because, noble gases only have van der wall’s radii while others have covalent radii and the van der wall’s radii are larger tham covalent radii. Thus, noble gases have large atomic size.
40. List the uses of neon and argon gases.
Ans. Uses of Neon
(i) Neon is used in discharge tubes and fluorescent bulbs for advertisement display purposes.
(ii) Neon bulbs are used in botanical gardens and in green houses.
(iii) Neon is used in voltage regulators and indicators.
Uses of Argon
(i) Argon is used for filling electric bulbs.
(ii) It is used to provide an inert atmosphere in high temperature metallurgical operations (or welding of metals or alloys).
(iii) Argon is used in rectifiers and in radio valves.
Share page on
NCERT Solutions Class 12 Chemistry
- Chapter 1 The Solid State
- Chapter 2 Solutions
- Chapter 3 Electrochemistry
- Chapter 4 Chemical Kinetics
- Chapter 5 Surface Chemistry
- Chapter 6 General Principles and Processes of Isolation of Elements
- Chapter 7 The p-Block Elements
- Chapter 8 The d-and f-Block Elements
- Chapter 9 Coordination Compounds
- Chapter 10 Haloalkanes & Haloarenes
- Chapter 11 Alcohols, Phenols and Ethers
- Chapter 12 Aldehydes, Ketones and Carboxylic Acids
- Chapter 13 Amines
- Chapter 14 Biomolecules
- Chapter 15 Polymers
- Chapter 16 Chemistry in Everyday Life
CBSE CLASS 12 NCERT SOLUTIONS
- NCERT Solutions Class 12 English Core
- NCERT Solutions Class 12 Physics
- NCERT Solutions Class 12 Chemistry
- NCERT Solutions Class 12 Biology
- NCERT Solutions Class 12 Business Studies
- NCERT Solutions Class 12 Mathematics
- NCERT Solutions Class 12 Accountancy
- NCERT Solutions Class 12 Economics
- NCERT Solutions Class 12 Geography
- NCERT Solutions Class 12 History
- NCERT Solutions Class 12 Political Science
CBSE CLASS 12 SYLLABUS
- CBSE Class 12 English core Syllabus
- CBSE Class 12 Mathematics Syllabus
- CBSE Class 12 Physics Syllabus
- CBSE Class 12 Chemistry Syllabus
- CBSE Class 12 Biology Syllabus
- CBSE Class 12 Accountancy Syllabus
- CBSE Class 12 Business Studies Syllabus
- CBSE Class 12 Economics Syllabus
- CBSE Class 12 History Syllabus
- CBSE Class 12 Geography Syllabus
- CBSE Class 12 Political science Syllabus
- CBSE Class 12 Sociology Syllabus
- CBSE Class 12 Psychology Syllabus
- CBSE Class 12 Physical education Syllabus
- CBSE Class 12 Applied mathematics Syllabus
- CBSE Class 12 History of Indian Arts Syllabus
CBSE CLASS 12 Notes
- CBSE Class 12 Physics Notes
- CBSE Class 12 Chemistry Notes
- CBSE Class 12 Biology Notes
- CBSE Class 12 Maths Notes
- CBSE Class 12 Accountancy Notes
- CBSE Class 12 Business Studies Notes
- CBSE Class 12 Economics Notes
- CBSE Class 12 History Notes
- CBSE Class 12 Geography Notes
- CBSE Class 12 Political Science Notes
