NCERT Solutions for Class 12 Chemistry Chapter 10 - Haloalkanes & Haloarenes
NCERT Solutions for Class 12 Chemistry Chapter 10 Free PDF Download
Please Click on Free PDF Download link to Download the NCERT Solutions for Class 12 Chemistry Chapter 10 Haloalkanes & Haloarenes
To download the complete Syllabus (PDF File), Please fill & submit the form below.
1. Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl, or aryl halides:
(i) (CH3)2CHCH(Cl)CH3
(ii)CH3CH2CH(CH3)CH(C2H5)CI
(iii) CH3CH2C(CH3)2CH2I
(iv) (CH3)3CCH2CH(Br)C6H5
(v) CH3CH(CH3)CH(Br)CH3
(vi) CH3C(C2H5)2CH2Br
(vii) CH3C(Cl)(C2H5)CH2CH3
(viii) CH3CH = C(Cl)CH2CH(CH3)2
(ix) CH3CH = CHC(Br)(CH3)2
(x) P–ClC6H4CH2CH(CH3)2
(xi) m-ClCH2C6H4CH2C(CH3)3
(xii) o-Br -C6H4CH (CH3)CH2CH3
Ans. (i) 2-Chloro-3methylbutane, 2° alkyl halide
(ii) 3-Chloro-4methyl hexane, 2° alkyl halide
(iii) 1-Iodo-2,2-dimethylbutane, 1° alkyl halide
(iv) l-Bromo-3, 3-dimethyl-1-phenylbutane, 2° benzylic halide
(v) 2-Bromo-3-methylbutane, 2° alkyl halide
(vi) 1-Bromo-2-ethyI-2-methylbutane, 1° alkyl halide
(vii) 3-Chloro-3-methylpentane, 3° alkyl halide
(viii) 3-Chloro-5-methylhex-2-ene, vinylic halide
(ix) 4-Bromo-4-methylpent-2-ene, allylic halide
(x) 1-Chloro-4-(2-methylpropyl) benzene, aryl halide
(xi) 1-Chloromethyl-3-(2,2-dimethylpropyl) benzene, 1° benzylic halide.
(xii) 1-Bromo-2-(l-methylpropyl) benzene,aryl halide.
2. Give the IUPAC names of the following compounds:
(i) CH3CH(Cl)CH (Br)CH3
(ii) CHF2CBrCIF
(iii) ClCH2C – CCH2Br
(iv) (CCl3)3CCl
(v) CH3C(p-ClC6H4)2CH(Br)CH3
(vi) (CH3)3CCH = C(Cl)C6H4I-p
Ans. (i) 2-Bromo-3-chlorobutane
(ii) 1-Bromo-1-chloro-1,2,2-trifluoroethane
(iii) 1-Bromo-4-chlorobut-2-yne
(iv) 2-(Trichloromethyl)-1, 1,1,2,3,3,3-heptachloropropane
(v) 2-Bromo-3,3-bis-(4-chlorophenyl) butane
(vi) 1-Chloro-1-(4-iodophenyl)-3,3- dimethylbut-1-ene.
3. Write the structures of the following organic halogen compounds:
(i) 2-ChIoro-3-methylpentane
(ii) p-Bromochlorobenzene
(iii) 1-Chloro-4-ethylcyclohexane
(iv) 2-(2-Chlorophenyl)-1-iodooctane
(v) 2-Bromobutane
(vi) 4-tert-Butyl-3-iodoheptane
(vii) 1-Bromo-4-sec-butyl-2-methylbenzene
(viii) 1,4-Dibromobut-2-ene
Ans.

4. Which one of the following has the highest dipole moment?
(i) CH2Cl2 (ii) CHCl3 (iii) CCl4
Ans. The three dimensional structures of the three compounds along with the direction of dipole moment in each of their bonds are given below:

5. A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
Ans. The hydrocarbon with molecular formula C5H10 can either a cycloalkane or an alkene.
Since the compound does not react with Cl2 in the dark, therefore it cannot be an alkene but must be a cycloalkane. Since the cycloalkane reacts with Cl2 in the presence of bright sunlight to give a single monochloro compound, C5H9Cl, therefore, all the ten hydrogen atoms of the cycloalkanes must be equivalent. Thus, the cycloalkane is cyclopentane.

6. Write the isomers of the compound having formula C4H9Br.
Ans. There are 4 isomers of C4H9Br as given below:

7. Write the equations for the preparation of 1-iodobutane from
(i) 1-butanol
(ii) 1-chlorobutane
(iii) But-l-ene.
$$\textbf{Ans.}\space\text{(i)}\space\underset{1-\text{butanol}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{OH + Kl + H}_{3}\text{PO}_{4}}\xrightarrow{}\underset{1-\text{iodobutane}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{I}+\text{H}_{2}\text{O}+\text{KH}_{2}\text{PO}_{4}}\\\text{(ii)}\space\underset{1-\text{chtorobutane}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{Cl + Kl}}\xrightarrow{\text{Acetone}}\underset{1-\text{iodobutane}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{I + KCl}\darr}\\\underset{\text{But-1-ene}}{\text{(iii)}\space\text{CH}_{3}\text{CH}_{2}-\text{CH = CH}_{2}+\text{HBr}}\xrightarrow{\text{Peroxide}}\underset{\text{1- bromobutane}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{Br}}\xrightarrow{\text{Nal/Acetone}}\underset{\text{1-iodobutane}}{\text{CH}_3\text{CH}_2\text{CH}_2\text{CH}_2-\text{I}+\text{NaBr}}$$
8. What are ambident nucleophiles? Explain with an example.
Ans. The nucleophiles which has two different electron donor atoms and can attack through two different sites are called ambident nucleophiles. For example, cyanide ion is a resonance hybrid of the following two structures:
It can attack through carbon to form cyanide and through N to form is O cyanide.
9. Which compound in each of the following-pairs. Will react faster in SN2 reaction with –OH?
(i) CH3Br or CH3I
(ii) (CH3)3CCl or CH3Cl
Ans. (i) CH3—I will react faster because bond dissociation enthalpy of C—I bond is less than that of C—Br bond.
(ii) CH3Cl will react faster because of less stearic hindrance as compared to (CH3)3CCl.
10. Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
(i) 1-Bromo-1-methylcyclohexane.
(ii) 2-Chloro-2-methylbutane.
(iii) 2,2,3-Trimethyl-3-bromopentane.
Ans.

11. How will you bring about the following conversions?
(i) Ethanol to but-1-yne.
(ii) Ethane to bromoethene
(iii) Propene to 1-nitropropane
(iv) Toluene to benzyl alcohol
(v) Propene to propyne
(vi) Ethanol to ethyl fluoride
(vii) Bromomethane to propanone
(viii) But-1-ene to but-2-ene
(ix) 1-Chlorobutane to n-octane
(x) Benzene to biphenyl
Ans.
$$\underset{\text{Ethanol}}{\text{CH}_{3}\text{CH}_{2}\text{OH}}\xrightarrow[-\text{SO}_{2},-\text{HCl}]{\text{SOCl}_{2},\text{Pyridine}}\underset{\text{Chloroethane (I)}}{\text{CH}_{3}\text{CH}_{2}-}\text{Cl}+\text{SO}_{2}\uparrow+\text{HCl}\uparrow\\\text{CH}≡\text{CH}+\text{NaNH}_{2}\xrightarrow[]{\text{Liq.\text{NH}}_{3},196\space\text{K}}\underset{\text{Sodium acetylide}}{\text{HC}≡\text{C}^{\normalsize-}\text{Na}^{\normalsize+}}\\\underset{\text{(I)}}{\text{CH}_{3}-\text{CH}_{2}-\text{Cl}+}\underset{\text{(II)}}{\text{HC}}≡\text{C}^{\normalsize-}\text{Na}^{\normalsize+}\xrightarrow{}\underset{\text{But-1-yne}}{\text{CH}_{3}\text{CH}_{2}-\text{C}≡\text{CH + NaCl}}\\\text{(ii)}\space\text{CH}_{3}-\text{CH}_{3}+\text{Br}_{2}\xrightarrow{\text{hv, 520-670\text{K}}}\underset{\text{Bromoethane}}{\text{CH}_{3}\text{CH}_{2}-\text{Br + HBr}}\xrightarrow[\text{-\text{HBr}}]{\text{KOH}(alc)}\text{CH}_{2}=\text{CH}_{2}\xrightarrow{\text{Br}_{2}/\text{CCl}_{4}}\text{BrCH}_{2}\text{CH}_{2}\text{Br}\xrightarrow[-\text{HBr}]{\Delta/\text{KOH}(\text{alc})}\underset{}{\text{CH}_{2}=\text{CHBr}}\\\text{(iii)\space}\underset{\text{Propene}}{\text{CH}_{3}-\text{CH}=\text{CH}_{2}}\xrightarrow[\text{Peroxide effect}]{\text{HBr, ROOR}}\underset{1-\text{Bromopropane}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{Br}}\xrightarrow{\text{AgNO}_{2},\text{C}_{2}\text{H}_{5}\text{OH/\text{H}}_{2}\text{O}}\underset{1-\text{nitroprpane}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{NO}_{2}}$$


12. Explain why
(i) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
(ii) Alkyl halides, though polar, are immiscible with water?
(iii) Grignard reagents should be prepared under anhydrous conditions?
Ans. (i) Due to Sp2 hybridisation of C-atom in chlorobenezens, C-atom is more electronegative (greater s-character), whereas in cyclohexyl chloride, C-atom is in Sp3 hybridisation i.e., less electronegative (lesser s-chararter). So, polarity of C—Cl bond in chlorobenzene is less than the C—Cl bond in cyclohexyl chloride. Therefore, the dipole moments of chlorobenzene is lower than that of cyclohexyl chloride.
(ii) Water molecules have enough strong H-bonding which is difficult to be broken by alkyl halides, though they are also polar in nature. Therefore, alkyl halides, do not dissolve in water and form separate layers.
(iii) Grignards reagents (R-Mg-X) are readily decomposed
by water to produce alkanes. That is why they should be prepared under anhydrous conditions. Instead, ether is used as a solvent during the preparation of Grignard reagents.
$$\text{R — Mg — X + H}_2\text{O}\xrightarrow{}\underset{\text{Alkane}}{\text{R-H}+\text{Mg(OH)X}}$$
13. Give the uses of freon 12, DDT, carbon tetrachloride, and iodoform.
Ans. Use of freon 12:
1. It is used as a refrigerant
2. It is used as propellants in aerosols and foams
3. It is used for air conditioning purposes
Use of DDT:
1. It is used as a insecticide.
2. It is effective against mosquito and lice.
Use of carbon tetrachloride:
1. It is used in manufacturing refrigerants and propellants of aerosol cans.
2. It is used as feedstock in the synthesis of chlorofluro cartons and other chemicals.
3. It is used as cleansing agent in home and industries.
Use of iodoform:
1. It is used as an antiseptic, particularly for dressing wounds.
14. Write the structure of the major organic product in each of the following reactions:
$$\textbf{(i)\space \textbf{CH}}_{3}\textbf{CH}_{2}\textbf{CH}_{2}\textbf{Cl + Nal}\xrightarrow[\textbf{Heat}]{\textbf{Acetone}}\\\textbf{(ii)\space (CH}_{3})_{3}\textbf{CBr + KOH}\xrightarrow[\textbf{Heat}]{\textbf{Ethanol}}\\\textbf{(iii)\space CH}_{3}\textbf{CH(Br) CH}_{2}\textbf{CH}_{3}+\textbf{NaOH}\xrightarrow{\textbf{water}}\\\textbf{(iv)\space CH}_{3}\textbf{CH}_{2}\textbf{Br + KCN}\xrightarrow{\textbf{aq. ethanol}}\\\textbf{(v)\space}\text{C}_{6}\text{H}_{5}\textbf{ONa + C}_{2}\text{H}_{5}\textbf{Cl}\xrightarrow{}\\\textbf{(vi)\space CH}_{3}\textbf{CH}_{2}\textbf{CH}_{2}\textbf{OH + SOCl}_{2}\xrightarrow{}\\\textbf{(vii)\space CH}_{3}\textbf{CH}_{2}\textbf{CH}=\textbf{CH}_{2} + \textbf{HBr}\xrightarrow{\textbf{Peroxide}}\\\textbf{(viii)\space CH}_{3}\textbf{CH}=\textbf{C(CH}_{3}\textbf{)}_{2}+\textbf{HBr}\xrightarrow{} $$
$$\textbf{Ans.}\space(i)\space\underset{1-\text{Chloropropane}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{Cl} + \text{Nal}}\xrightarrow[\text{(Finkelstein)}]{\text{acetone, heat}}\underset{\text{1- iodopropane}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{I} + \text{NaCl}} $$
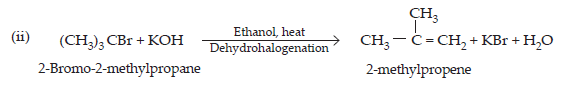

$$\text{(iv)\space CH}_{3}\text{CH}_{2}\text{Br + KCN}\xrightarrow[\text{(Nucleophilic substitution)}]{\text{aq. ethanol}}\underset{\text{Ethyl cyanide}}{\text{CH}_{3}-\text{CH}_{2}-\text{C}≡\text{N + KBr}}\\\underset{\text{Sodium phenoxide}}{\text{(v)\space C}_{6}\text{H}_{5}\text{O}^{\normalsize-}\text{Na}^{\normalsize+}} + \underset{\text{Ethylchloride}}{\text{C}_{2}\text{H}_{5}\text{Cl}}\xrightarrow[\text{synthesis}]{\text{Williamson's}}\underset{\text{Phenyl ethyl ether}}{\text{C}_{6}\text{H}_{5}-\text{O}-\text{C}_{2}\text{H}_{5}+\text{NaCl}}\\\text{(vi)\space}\underset{\text{Propan-1-ol}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{OH}} + \text{SOCl}_{2}\xrightarrow[\text{substitution}]{\text{Nucleophilic}}\underset{\text{1- Chloroprpane}}{\text{CH}_{3}-\text{CH}_{2}-\text{CH}_{2}-\text{Cl + HCl + SO}_{2}}\\\text{(vii)\space}\underset{\text{But-1-ene}}{\text{CH}_{3}\text{CH}_{2}\text{CH}=\text{CH}_{2} + \text{HBr}}\xrightarrow[\text{(Anti- Markownnikoff's addition)}]{\text{Peroxide}}\underset{\text{1- Bromobutane}}{\text{CH}_{3}-\text{CH}_{2}-\text{CH}_{2}-\text{CH}_{2}\text{Br}}$$

15. Write the mechanism of the following reaction:
$$\textbf{n-BuBr + KCN}\xrightarrow{\textbf{EtOH-H}_{2}\textbf{O}}\textbf{nBuCN}$$
Ans. KCN is a resonance hybrid of the following two contributing structures:
Thus, CN– ion is an ambident nucleophile. Therefore, it can attack the “carbon atom of C—Br bond in n-BuBr either through C or N. Since C—C bond is stronger than C—N bond, therefore, attack occurs through C to form n-butyl cyanide.

16. Arrange the compounds of each set in order of reactivity towards SN2 displacement:
(i) 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane.
(ii) 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 3-Bromo-2-methylbutane.
(iii) 1-Bromobutane,1-Bromo-2,2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methyl butane.
Ans. The SN2 reactions reactivity is affected by steric hindrance. More the steric hindrance slower the reaction.Thus, the order of reactivity will be 1° > 2° > 3°.

1-Bromopentane > 2-Bromopentane > 2-Bromo-2-methylbutane

Since in case of 1° alkyl halides steric hindrance increases in the order n-alkyl halides, alkyl halides with a substituent at any position other than the β-position, one substituent at the β-position, two substituents at the β-position, therefore, the reactivity decreases in the same order. Thus, the reactivity of the given alkyl bromides decreases as: 1-Bromobutane > 1-Bromo-3-methylbutane > 1-Bromo-2-methylbutane > 1-Bromo-2,2-dimethyl propane.
17. Out of C6H5CH2Cl and C6H5CHClC6H5 which is more easily hydrolysed by aqueous KOH.
Ans.

In SN1 reaction, reactivity depends upon the stability of carbonations. C6H5 — CH — C+6H5 carbonation is more stable as compared to the C6H5 C+H2. Therefore, C6H5CHClC6H5 get hydrolysed more easily than C6H5CHCl.
18. p-dichlorobenzene has higher m.p. than those of o- and m-isomers. Discuss.
Ans. The three isomers are position isomers which differ in the relative positions of the chlorine atoms in the ring:

As we know, p-isomer is more symmetrical as compared to the other isomers. This means that in the crystal lattice, molecules of the p-isomers are more closely packed as compared to the other isomers. As a result, it has a higher melting point and lower solubility as compared to ortho and meta isomers.
Haloarenes are less polar than haloalkanes and are insoluble in water. This is because of lack of hydrogen bonding. As a result, the attractive forces in haloarenes—water system remain less than the attractive forces in H2O molecules which are hydrogen bonded. Haloarenes are soluble in organic solvents of low polarity such as benzene, ether, chloroform, carbon tetrachioride etc.
19. How the following conversions can be carried out:
(i) Propene to propan-1-ol
(ii) Ethanol to but-1-yne
(iii) 1-Bromopropane to 2-bromopropane
(iv) Toluene to benzyl alcohol
(v) Benzene to 4-bromonitrobenzene
(vi) Benzyl alcohol to 2-phenylethanoic acid
(vii) Ethanol to propanenitrile
(viii) Aniline to chlorobenzene
(ix) 2-Chlorobutane to 3,4-dimethylhexane
(x) 2-Methyl-1-propene to 2-chloro-2-methylpropane.
(xi) Ethyl chloride to propanoic acid
(xii) But-1-ene to n-butyliodide
(xiii) 2-Chlropropane to 1-propanol
(xiv) Isopropyl alcohol to iodoform
(xv) Chlorobenzene to p-nitrophenol
(xvi) 2-Bromopropane to 1-bromopropane
(xvii) Chloroethane to butane,
(xviii) Benzene to diphenyl
(xix) tert-Butyl bromide to isobutyl bromide
(xx) Aniline to phenylisocyanide
Ans.
$$\text{(i)}\space\underset{\text{Propene}}{\text{CH}_{3}\text{CH}=\text{CH}_{2}}\xrightarrow{\text{HBr/Peroxide}}\underset{\text{1- bromopropane}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{Br}}\xrightarrow[\text{Hydrolysis}]{\text{Aq.KOH,}\Delta}\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{OH}\\\underset{\text{Ethanol}}{\text{(ii)\space CH}_{3}\text{CH}_{2}\text{OH}}\xrightarrow[\text{Pyridine}]{\text{SO}_{2}\text{Cl}_{2}}\underset{\text{Chloroethane}}{\text{CH}_{3}\text{CH}_{2}\text{Cl}}\xrightarrow[\text{-NaCl}]{{\text{CH≡CNa}}}\underset{\text{But-1-yen}}{\text{CH}_{3}\text{CH}_{2}\text{C≡ CH}}\\\text{(iii)}\space\underset{1-\text{bromopropane}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{Br}}\xrightarrow[\text{\text{Dehydration}}]{\text{KOH(alc),}\Delta}\underset{\text{Propane}}{\text{CH}_{3}\text{CH}_{2}\text{C}≡\text{CH}_{2}}\xrightarrow[\text{Mark, addition}]{\text{HBr}}\underset{\text{2-Bromopropane}}{\text{CH}_{3}-\text{CHBr}-\text{CH}_{3}}$$

$$\underset{\text{Ethanol}}{\text{(vii)\space CH}_{3}\text{CH}_{2}\text{OH}}\xrightarrow{\text{P/I}_{2}\Delta}\underset{\text{Iodoethane}}{\text{CH}_{3}\text{CH}_{2}\text{I}}\xrightarrow[\text{(Nucleophilic substitution)}]{\text{KCN, Et-OH-H}_{2}\text{O}}\underset{\text{Propanenitrile}}{\text{CH}_{3}\text{CH}_{2}\text{CN}}$$

$$\underset{\text{Ethyl chloride}}{\text{(xi)\space}\text{CH}_{3}\text{CH}_{2}\text{Cl}}\xrightarrow[\text{(Nucleophilic substitution)}]{\text{KCN, EtOH-H}_{2}\text{O}}\underset{\text{Propanenitrile}}{\text{CH}_{3}\text{CH}_{2}\text{CN}}\xrightarrow[\text{Hydrolysis}]{\text{H}^{\normalsize+}/\text{H}_{2}\text{O}}\underset{\text{Propanoic acid}}{\text{CH}_{3}\text{CH}_{2}\text{COOH}}\\\text{(xii)\space}\underset{\text{But-1-ene}}{\text{CH}_{3}\text{CH}_{2}\text{CH}=\text{CH}_{2}}\xrightarrow[\text{Anti-Mark, Addition}]{\text{HBr/R COOR}}\underset{\text{n-butylbromide}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{Br}}\xrightarrow[\text{Fin Kelstein reaction}]{\text{Nal, acetone}}\underset{\text{n-Butyliodide}}{\text{CH}_{3}\text{CH}_{2}\text{CH}_{2}\text{CH}_{2}\text{I}}$$


20. The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in presence of alcoholic KOH, alkenes are major products. Explain.
Ans. In aqueous medium KOH dissociates completely into OH– ions. These are (OH– ions) are strong nucleophiles which produces alcohols from alkyl halides by substitution reaction.
$$\underset{\text{Alkyl chloride}}{\text{R-Cl} + \text{KOH}(aq)}\xrightarrow{}\underset{\text{Alcohol}}{\text{R-OH + KCl}}$$
On the other hand an alcoholic solution of KOH contains alkoxide (RO–) ion, which is a strong base. Thus, it can abstract a hydrogen from the β-carbon of the alkyl chloride and from an alkene by eliminating a molecule of HCl.
$$\underset{\text{Alkyl chloride}}{\text{R - CH}_{2}-\text{CH}_{2}-\text{Cl + KOH (alc)}}\xrightarrow{}\underset{\text{Alkene}}{\text{R-CH = CH}_{2}+\text{KCl}+\text{H}_{2}\text{O}}$$
OH– ion is a much weaker base than RO– ion. Also, OH– ions is highly solvated in an aqueous solution and as a result, the basic character of OH– ion decreases. Therefore, it cannot abstract a hydrogen from the β-carbon.
21. Primary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b) Compound (b) is reacted, with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it give compound (d), C8H18 which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
Ans. (i) There are two primary alkyl halides having the molecular formula, C4H9Br.

(ii) Since compound (a) when reacted with Na metal gave a compound (d) with molecular formula C8H18 which was different from die compound obtained when n-butyl bromide was reacted with Na metal, therefore, (a) must be isobutyl bromide and compound (d) must be 2,3-dimethylhexane.
$$\text{2CH}_{3}-\text{CH}_{2}-\text{CH}_{2}-\text{CH}_{2}-\text{Br + 2Na}\xrightarrow[\text{Ether}]{\text{Wurtz reaction}}\underset{\text{n-Octane}}{\text{CH}_{3}-\text{CH}_{2}-\text{CH}_{2}-\text{CH}_{2}-\text{CH}_{2}-\text{CH}_{2}-\text{CH}_{2}-\text{CH}_{3}}$$

(iii) If compound (a) is isobutyl bromide, than the compound (b) which it gives on treatment with alcoholic KOH must be 2-methyl-1-propane.

(iv) The compound (b) on treatment with HBr gives compound (c) in accordance with Markownikoff rule. Therefore, compound (c) is tert-butyl bromide which is an isomer of compound (a), i.e., isobutyl bromide.

Thus,
(a) is isobutyl bromide,
(b) is 2-methyl-1-propane,
(c) is tert-butylbromide, and
(d) is 2,5-dimethylhexane.
22. What happens when a:
(i) n-butyl chloride is treated with alcoholic KOH.
(ii) Bromobenzene is treated with Mg in the presence of dry ether.
(iii) Chlorobenzene is subjected to hydrolysis.
(iv) Ethyl chloride is treated with aqueous KOH.
(v) Methyl bromide is treated with sodium in the presence of dry ether.
(vi) Methyl chloride is treated with KCN.
Ans. (i) When n-butyl chloride is treated with alcoholic KOH, the formation of but-1-ene takes place. This reaction is a dehydrohalogenation reaction.
$$\underset{\text{n-Butyl chloride}}{\text{CH}_{3}-\text{CH}_{2}-\text{CH}_{2}-\text{CH}_{2}-\text{Cl}+\text{KOH (alc)}}\xrightarrow[\text{Dehydrohalogenation}]{\Delta}\underset{\text{But-1-ene}}{\text{CH}_{3}-\text{CH}_{2}-\text{CH}=\text{CH}_{2} + \text{KCl + H}_{2}\text{O}}$$
(ii) When bromobenzene is treated with Mg in the presence of dry ether , phenylmagnesium bromide is formed.

(iii) The hydrolysis of chlorobenzene is not possible under normal conditions. In order to subject chlorobenzene for hydrolysis, we need to heat chlorobenzene in an aqueous medium with NaOH solution at temperature 623K and a pressure of 300 atm to form phenol.

(iv) When ethyl chloride is treated with aqueous KOH, it undergoes hydrolysis to form ethanol.
$$\underset{\text{Ethyl chloride}}{\text{CH}_{3}\text{CH}_{2}-\text{Cl + KOH (aq)}}\xrightarrow[\Delta]{\text{Hydrolysis}}\underset{\text{Ethyl alcohol}}{\text{CH}_{3}\text{CH}_{2}-\text{OH} + \text{KCl + H}_{2}\text{O}}$$
(v) When methyl bromide is treated with sodium in the presence of dry ether, ethane is formed. This reaction is known as the Wurtz reaction.
$$\underset{\text{Methyl bromide}}{2\text{CH}_{3}-\text{Br + 2Na}}\xrightarrow[\text{(wurtz reaction)}]{\text{Dry ether}}\underset{\text{Etahne}}{\text{CH}_{3}-\text{CH}_{3} + 2\text{NaBr}}$$
(vi) When methyl chloride is treated with KCN, it undergoes a substitution reaction to give methyl cyanide.
$$\underset{\text{Methyl chloride}}{\text{CH}_{3}-\text{Cl + KCN}}\xrightarrow[\text{Nucleophilic substitution}]{\text{Et-OH-H}_{2}\text{O},\Delta}\underset{\text{Methyl cyanide}}{\text{CH}_{3}\text{C}≡\text{N} + \text{KCl}}$$