NCERT Solutions for Class 9 Science Chapter 2 - Is Matter Around us Pure
NCERT Solutions for Class 9 Science Chapter 2 Free PDF Download
Please Click on Free PDF Download link to Download the NCERT Solutions for Class 9 Chemistry Chapter 2 Is Matter Around us Pure
To download the complete Syllabus (PDF File), Please fill & submit the form below.
Q. What are pure substances?
Ans. Pure substances are elements and compounds with no impurities.
Q. The ‘sea-water’ can be classified as a homogeneous as well as heterogeneous mixture. Comment.
Ans. Sea-water can be classified as a homogeneous mixture because it contains salts dissolved in water. It can be classified as a heterogeneous mixture also since it contains mud, sand and decayed parts of plants.
Q. How would you confirm that a colourless liquid given to you is pure water?
Ans. As every liquid has a characteristic boiling point at 1 atmospheric pressure and if the given colourless liquid boils exactly at 373 K at 1 atmospheric pressure, then it is pure water. If the boiling point is different that means water is contaminated.
Q. Salt can be recovered from its solution by evaporation. Suggest some other technique for the same.
Ans. Salt solution can be recovered by heating and make it superheated solution. Crystallisation will occur after cooling and salt will separate out from the solution.
Q. Smoke and fog both are aerosols. In what way are they different?
Ans. In smoke: Dispersed phase is solid and dispersed medium is gas.
In fog: Dispersed phase is liquid and dispersed medium is gas.
Q. (i) Name the metal:
(a) Which can be easily cut with a knife?
(b) Which forms amalgams?
(c) Which has no fixed shape?
(d) Which has a low melting point?
(e) Which is yellow in colour?
(ii)Classify the following as chemical or physical changes:
(a) Cutting of trees
(b) Melting of butter in a pan
(c) Rusting of almirah
(d) Boiling of water to form steam
(e) Passing electric current through water and the water breaking down into hydrogen and oxygen gases
(f) Dissolving common salt in water
(g) Making a fruit salad with raw fruits
(h) Burning of paper and wood
(b) Mercury
(c) Mercury
(d) Sodium
(e) Gold
(ii)
| Physical Change | Chemical Change |
| (a) Cutting of trees | (c) Rusting of almirah |
| (b) Melting of butter in a pan | (e) Passing electric current through water and the water breaking down into hydrogen and oxygen gases |
| (d) Boiling of water to form steam | |
| (f) Dissolving common salt in water | |
| (g) Making a fruit salad with raw fruits | (h) Burning of paper and wood |
Q. (i) Which of the following can be separated by using a separating funnel and which cannot be separated by using a separating funnel? Give reason.
(a) Water and kerosene mixture
(b) Water and acetone mixture.
(ii)Classify the following into elements, compounds and mixtures.
(a) sodium
(b) soil
(c) sugar solution
(d) silver
(e) calcium carbonate
(f) tin
(g) silicon
(h) coal
(i) air
(j) soap
(k) methane
(l) carbon dioxide
(m) blood
Ans. (i) (a) Water and kerosene mixture can be separated by using a separating funnel because these are immiscible liquids and they have different densities.
(b) Water and acetone mixture cannot be separated by using a separating funnel because these are miscible liquids.
(ii)
| Elements | Sodium, silver, tin, silicon |
| Compounds | Calcium, carbonate, methane, carbon dioxide |
| Mixtures | Sugar solution, soil, coal, air, soap and blood |
Q. (i) Define the following :
(a) Sol
(b) Aerosol
(c) Emulsion
(d) Foam
Give one example of each.
(ii) How are sol, solution and suspension different from each other?
(iii) Explain why particles of a colloidal solution do not settle down when left undisturbed , while in the case of a suspension they do.
(a) Sol: Sol is a colloid in which tiny solid particles are dispersed in a liquid medium. Examples are ink and soap solution.
(b) Aerosol: Aerosol is a colloid in which a solid or liquid is dispersed in a gas. Examples are hairspray and fog.
(c) Emulsion: An emulsion is a colloid in which minute droplets of one liquid are dispersed in another liquid which is not miscible with it. Examples are milk and butter.
(d) Foam: A foam is a colloid in which a gas is dispersed in a liquid medium. Examples are soap bubbles and shaving cream.
(ii)
| Sol (colloid) | Solution | Suspension |
| Size of solute particles between 1nm to 100 nm. | Size of solute particles is less than 1nm. | Size of solute particles is more than 100 nm. |
| It is a stable solution. | It is stable. | It is unstable. |
| It scatters a beam of light that is called tyndall effect. | It does not scatter light. | It scatters a beam of light sometimes. |
| Solute particles pass through filter paper. | Solute particles pass through the filter paper. | Solute particles do not pass through the filter paper. |
(ii) In colloidal solutions, size of particles is smaller than the particles of suspension. Thus, due to gravity, particles settle down in suspension. But in colloidal solutions particles move in random motion due to which they do not settle down.
Q. (i) Differentiate between homogeneous and heterogeneous mixtures with examples.
OR
List the points of differences between homogeneous and heterogeneous mixtures.
(ii) Identify the following as homogeneous or heterogeneous mixture.
(a) Gasoline
(b) Dirt
(c) Smog
(d) Alcohol
(e) Iron nail
(f) Vinegar
(g) Aerosol spray
(h) Air
(i) Sea water
(j) Steel
Ans. (i) Difference between homogeneous and heterogeneous mixtures are:
| Homogeneous mixture | Heterogeneous mixture |
| It has uniform composition. | They do not have uniform composition throughout the solution. |
| No visible boundary of separation. | Shows visible boundaries of separation. |
| They consist of only one phase. Example: Mixture of sugar and water as sugar solution. | They consist of more than one phase. Example: Mixture of sugar and sand. |
(ii)(a) Gasoline : Homogeneous mixture
(b) Dirt: Heterogeneous mixture
(c) Smog: Heterogeneous mixture
(d) Alcohol : Homogeneous mixture
(e) Iron nail: Homogeneous mixture
(f)Vinegar: Homogeneous mixture
(g)Aerosol spray: Heterogeneous mixture
(h)Air: Homogeneous mixture
(i)Sea water: Homogeneous mixture
(j)Steel: Homogeneous mixture
Q. What would you observe when:
(i) A saturated solution of potassium chloride prepared at 60°C is allowed to cool at room temperature.
(ii) An aqueous sugar solution is heated to dryness
(iii) A mixture of iron filings and sulphur powder is strongly heated?
Ans. (i) Crystals of potassium chloride will separate out.
(ii)On heating sugar solution, water from solution will evaporate and residue after further heating will turn out black and sugar will get charred.
(iii)Iron sulphidewill be formed from heating of iron filings and sulphur.
Q. (i) Explain the method of separating a mixture containing camphor, salt and iron nails from the mixture.
(ii)Calculate the mass of sodium sulphate required to prepare its 20% (mass percent) solution in 100 g of water?
Ans. (i) A horse-shoe magnet is used to separate a mixture containing camphor, salt and iron nails when magnet is moved on the surface of the mixture of camphor, common salt and iron nails. The iron nails are attracted by the magnet, they cling to the poles of the magnet and get separated. This process is repeated a number of times till complete separation of iron nails occur leaving behind mixture of camphor and common salt. Mixture of camphor and common salt is heated. Camphor sublimes on heating leaving behind common salt which can be recovered in the form of sublimate by cooling its vapours.
(ii)Let the mass of sodium sulphate required to prepare the solution be “x” grams. Given, Mass of the solvent (water) = 100 g.
Mass of the solution = (x +100) gx g of solute (sodium sulphate) is dissolved in(x + 100) g of solution
$$20\%=\frac{x}{x+100}× 100\\ 20x + 2000 = 100x\\ 80x = 2000\\ x = 25 g$$
Thus, 25 g of sodium sulphate will be required to prepare 20% solution in 100 g water.
Q. A child wanted to separate the mixture of dyes constituting a sample of ink. He marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in Fig. The filter paper was removed when the water moved near the top of the filter paper.
(i)What would you expect to see, if the ink contains three different coloured components?
(ii)Name the technique used by the child.
(iii)Suggest one more application of this technique.

Ans. (i) If the ink contains three different coloured components, three different bands will be seen on the filter paper.
(ii)Paper chromatography.
(iii)Paper chromatography can be used to separate the pigments present in chlorophyll.
Q. (i) To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
(ii)Name the technique to separate:
(a) Butter from curd
(b) Salt from sea-water
(c) Camphor from salt
Ans. (i) Given, mass of solute (sodium chloride) = 36 g
Mass of solvent(water) = 100 g
Then, mass of solution = mass of solute + mass of solvent
= (36 + 100) g
= 136 g
$$\text{Thus,concentration of the solution (mass by mass percentage)}=\frac{Mass of solute}{Mass of solvent}\\ =\frac{36}{136}× 100 = 26.4 \%\\ \text{Thus, the concentration of saturated solution at }293K\space is\space 26.4\%.$$
(ii)
(a) Centrifugation
(b) Evaporation
(c) Sublimation
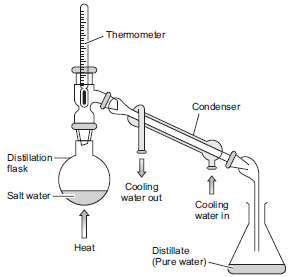
Q. (i) How can you obtain pure water from a salt-water mixture (or salt-solution)? Draw a neat and labelled diagram of the apparatus you would use to obtain pure water from a salt-water mixture (or salt-solution).
(ii) A group of students took an old shoe box and covered it with a black paper from all sides. They fixed a source of light (a torch) at one end of the box by making a hole in it and made another hole on the other side to view the light. They placed a milk sample contained in a beaker/tumbler in the box as shown in the figure.
They were amazed to see that milk taken in the tumbler was illuminated. They tried the same activity by taking a salt solution but found that light simply passed through it?

(a) Explain why the milk sample was illuminated. Name the phenomenon involved.
(b) Same results were not observed with a salt solution. Explain.
(c) Can you suggest two more solutions which would show the same effect as shown by the milk solution?
flask and heated. Some porcelain pieces are put in the distillation flask to avoid bumping of the solution due to uneven heating. On heating, water forms vapours which rise up and come out through the side tube of the distillation flask, and go into water condenser. Cold water from tap is circulated through the outer tube of condenser for cooling the vapours. The hot vapours get cooled in the condenser to form pure water (i.e. distilled water) which trickles down from the condenser and collects in the beaker. Since the salt is non-volatile, so it remains behind in the distillation flask.
(ii)
(a) Milk is a colloid. The particulate matter present inside milk scatter the light passing through milk and shows Tyndall effect.
(b) Salt solution is a homogeneous solution. Small particles present in a salt solution do not scatter light and hence, a salt solution does not exhibit Tyndall effect.
(c) Detergent solution and
Q. (i) Discuss a method to separate a mixture of common salt and sand.
(ii)When 23.5 g of sodium chloride dissolves in 60 g of water at 25°C. Calculate the solubility of sodium chloride in water at that temperature.
(iii) Give some examples of Tyndall effect observed in your surroundings.
Q. (i) Define heterogeneous mixtures.
(ii)How is blood a heterogeneous substance?
(iii)The sea-water can be classified as a homogeneous mixture. Comment.
(ii)Name the method used to separate iodine from a mixture of iodine and common salt. Also explain it.
(iii)Which separation techniques will you apply for the separation of the following?
(a) Sodium chloride from its solution in water.
(b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride.
(c) Small pieces of metal in the engine oil of a car.
(d) Different pigments from an extract of flower petals.
(e) Butter from curd.
(f)Oil from water.
(g)Tea leaves from tea.
(h)Iron pins from sand.
(i)Wheat grains from husk.
(j)Fine mud particles suspended in water.